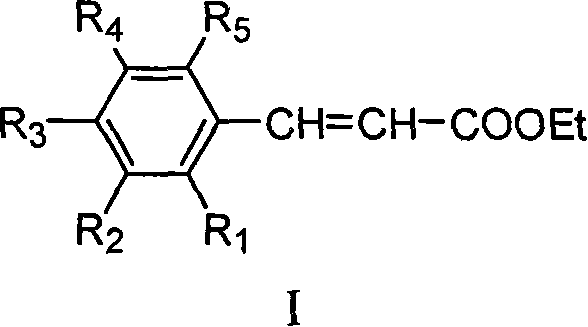
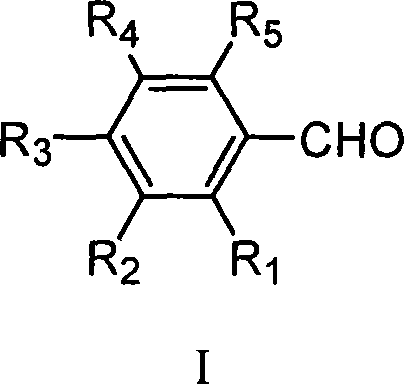
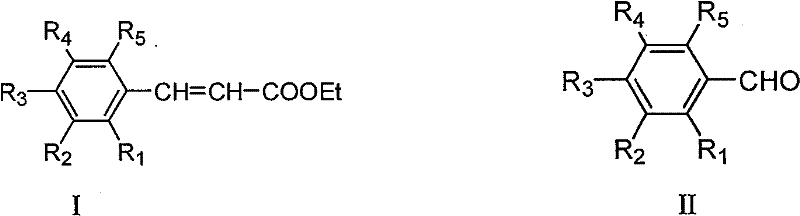Method for preparing ethyl cinnamate derivative
A technology of ethyl cinnamate and monoethyl malonate, which is applied in the field of preparation of ethyl cinnamate derivatives, can solve the problem of no low-cost green synthesis process of ethyl cinnamate derivatives, and achieve high product yield , wide application range, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of ethyl p-methoxycinnamate
[0021] 1. Preparation of monoethyl malonate potassium salt: add 210mL diethyl malonate and 600mL absolute ethanol to a 2L three-necked flask; dissolve 86g potassium hydroxide in 800mL absolute ethanol, and add dropwise to the aforementioned Solution, drop within 1h; continue to stir for 2h, and then stand overnight.
[0022] 2. Preparation of monoethyl malonate: Add 73 mL of glacial acetic acid into the solution obtained in step (1), and stir for about 1 h at room temperature.
[0023] 3. Preparation of crude ethyl p-methoxycinnamate: add 100mL anisaldehyde and 12.5g glycine to the solution obtained in step (2), heat up to reflux (about 90°C-100°C) for 6 hours; recover most of the solvent After ethanol, 500 mL of distilled water was added and stirred properly, and then stood overnight at room temperature; filtered, and the filter cake was washed 3 times with distilled water to obtain 171 g of crude product.
[0024] 4. Refinin...
Embodiment 2
[0026] Preparation of Ethyl Ferulate
[0027] 1. Preparation of monoethyl malonate potassium salt: add 216mL diethyl malonate and 700mL absolute ethanol to a 2L three-necked flask; dissolve 90g potassium hydroxide in 800mL absolute ethanol, and add dropwise to the aforementioned Solution, drop within 1h; continue to stir for 2h, and then stand overnight.
[0028] 2. Preparation of monoethyl malonate: Add 75 mL of glacial acetic acid into the solution obtained in step (1), and stir for about 1 h at room temperature.
[0029] 3. Preparation of ethyl ferulate crude product: add 122g vanillin and 12g glycine to the solution obtained in step (2), heat up to reflux reaction for 8h; after recovering most of the solvent ethanol, add 600mL distilled water and stir properly, then Stand overnight at room temperature; filter, and wash the filter cake twice with distilled water to obtain 127 g of crude product.
[0030] 4. Refining of ethyl ferulic acid: the crude product obtained in ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com