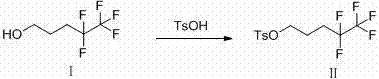
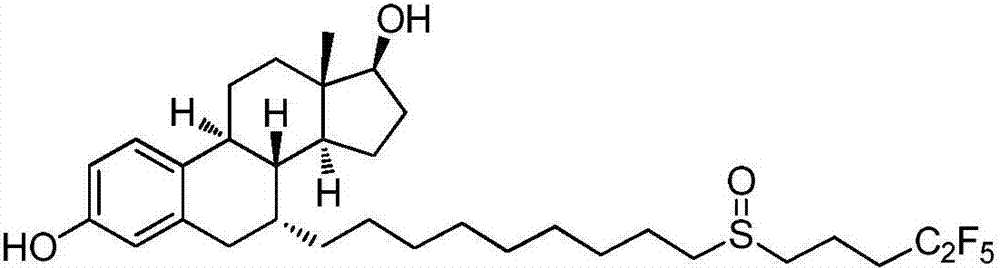
Synthetic method of fulvestrant side chain intermediate
A technology of fulvestrant and its synthesis method, which is applied in the field of drug synthesis, can solve problems such as unfavorable industrial production, pungent smell, and relatively large environmental impact, and achieve improved reagent use efficiency, avoiding environmental pollution, and production process operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of p-toluenesulfonic acid supported on molecular sieve
[0028] Weigh 100 g of 4A molecular sieve, soak in 1 L of 25% p-toluenesulfonic acid aqueous solution for 30 hours, and filter with suction. Dry it in an oven at 105°C for 8 hours, and put it in a desiccator after cooling for later use. The loading of p-toluenesulfonic acid was determined to be 35.6% by gravimetric method.
Embodiment 2
[0030] Add 20ml of pentafluoropentanol to the reaction flask, add 10g of p-toluenesulfonic acid supported on molecular sieve prepared according to Example 1, react at 65°C for 4 hours, filter and recover the molecular sieve, naturally cool and crystallize the filtrate, and recover pentafluoropentanol by filtration, the filter cake Dry to obtain 5.6 g of pentafluoropentanol p-toluenesulfonate, with a yield of 85% based on p-toluenesulfonic acid.
Embodiment 3
[0032] Add 30ml of pentafluoropentanol to the reaction flask, add 10g of p-toluenesulfonic acid loaded on molecular sieve prepared according to Example 1, react at 50°C for 6 hours, filter and recover the molecular sieve, naturally cool and crystallize the filtrate, and recover pentafluoropentanol by filtration, the filter cake After drying, 4.9 g of pentafluoropentanol p-toluenesulfonate was obtained, with a yield of 74% based on p-toluenesulfonic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com