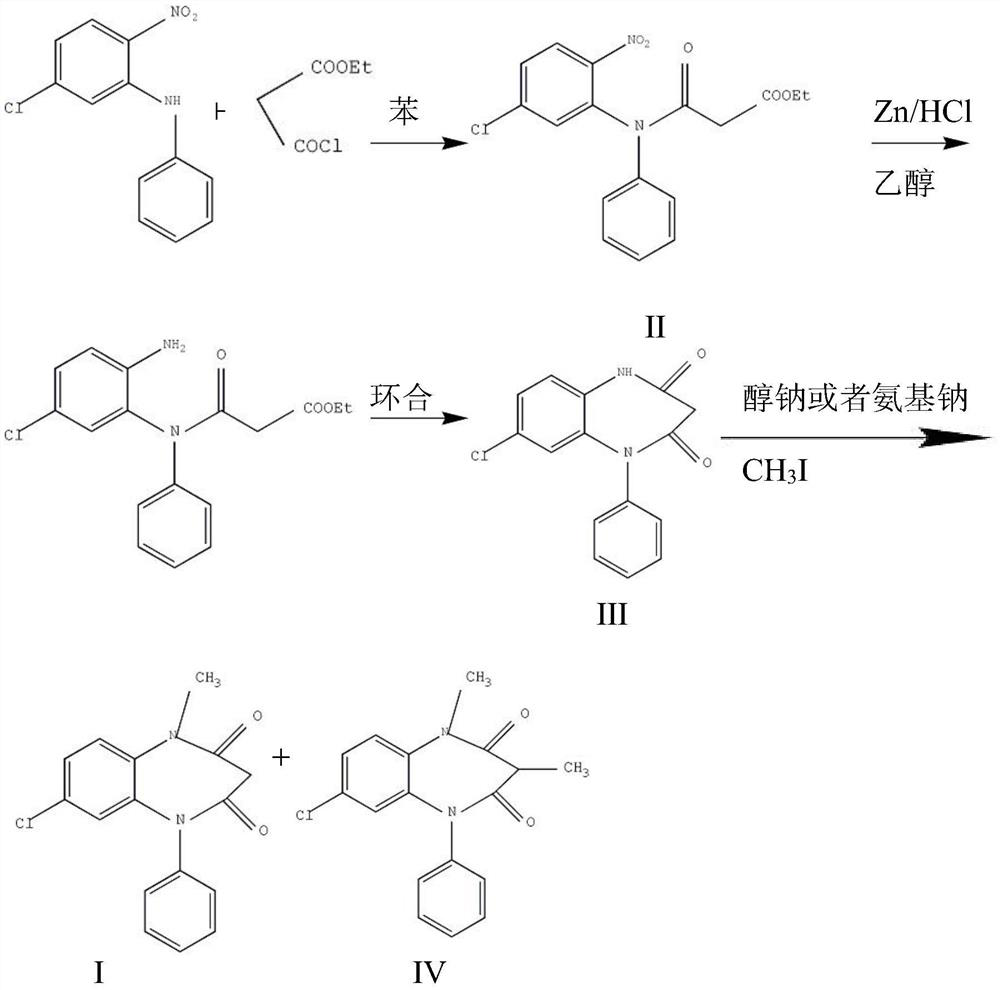
The preparation method of clobazam
A technology for clobazam and chlorodiphenylamine, which is applied in 1 field and can solve the problems of affecting the exertion and application of drug efficacy, not having an effect on epilepsy, and low purity of clobazam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] The preparation method of clobazam:
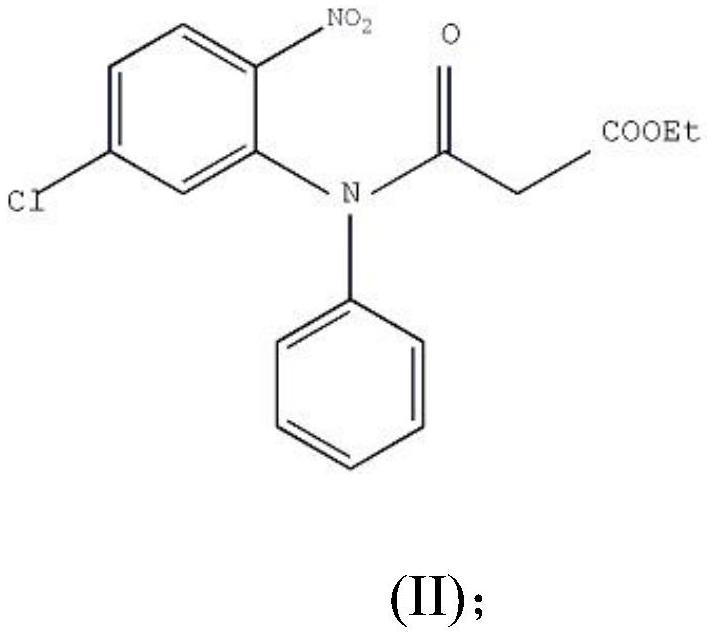
[0027] Step 1: Preparation of compound Ⅱ
[0028] In a 20L reaction flask, add 2.5kg of 2-nitro-5-chlorodiphenylamine, 16L of anhydrous acetonitrile, and 1.8kg of monoethyl malonate chloride, stir, and heat to reflux for 12 hours. Distill acetonitrile under reduced pressure at 60°C, add 8L of ethanol and stir to dissolve, cool and crystallize at 0-5°C, filter with suction, and dry at 60°C to obtain yellow crystals weighing 3.3kg. The melting point is 85-87°C, and the yield is 91%.
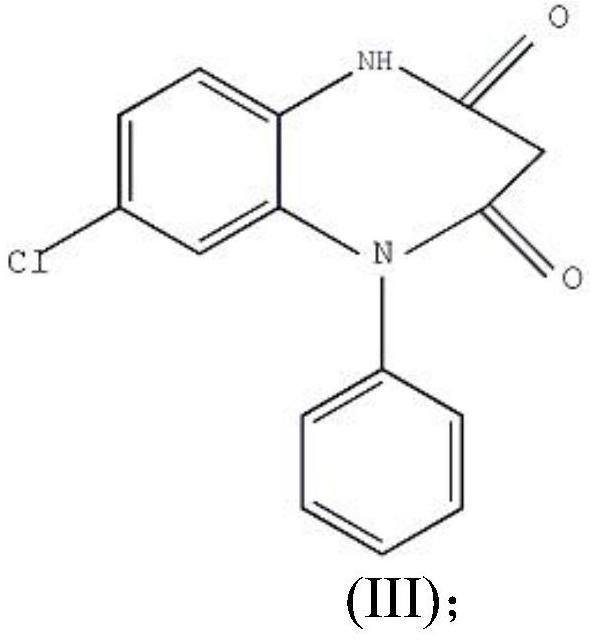
[0029] Step 2: Preparation of Compound III
[0030] In a 50L reaction bottle, add 3kg of crushed compound II that has passed through a 80-mesh sieve, stir with 30L of ethanol, and add 15L of hydrochloric acid dropwise at a temperature below 20°C. After the addition was completed within 1 hour, a white solid was precipitated. After the addition, the mixture was stirred for 4 hours, filtered with suction, washed with water until neutral, washed with eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com