Patents
Literature
49 results about "Nitrochlor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of paranitroaniline
ActiveCN102617361APromote safe productionIncrease production capacityOrganic compound preparationAmino compound preparationP-NitroanilineReaction temperature
Owner:苏州市罗森助剂有限公司
Method for preparing o-chloroaniline by virtue of solvent-free catalytic hydrogenation
ActiveCN103664641ASolve the problem of hydrogenolysis and dechlorinationReduce corrosionOrganic compound preparationAmino compound preparationPtru catalystSolvent free
The invention belongs to the technical field of fine chemical engineering, and relates to a method for preparing o-chloroaniline by virtue of solvent-free catalytic hydrogenation ortho-nitrochlorobenzene. The method is characterized in that ortho-nitrochlorobenzene is adopted as a raw material to be reacted with hydrogen in the presence of a catalyst at the temperature of 70-120 DEG C and the pressure of 0.5-5.0 MPa so as to obtain o-chloroaniline through the processing after the reaction. By adopting the method, no solvent is added, the defect for adding the solvent can be overcome, the environmental pollution problem and the solvent recycling problem can be avoided, the equipment investment is reduced, the production cost is reduced, the conversion rate of the ortho-nitrochlorobenzene can reach 100 percent, the selectivity of the o-chloroaniline is more than 99.2 percent, and the dechlorinating rate is less than 0.09 percent.
Owner:CHINA PETROLEUM & CHEM CORP +1
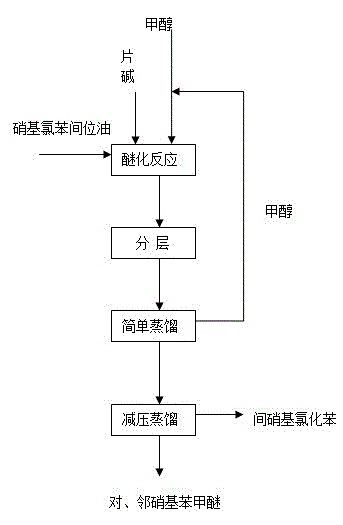
Method for preparing nitroanisole from m-nitrochlorobenzene oil
ActiveCN104557557AShort reaction timeThe reaction cycle is shortenedOrganic chemistryOrganic compound preparationChlorobenzeneP-nitroanisole
The invention aims to provide a nitroanisole production method with low cost, low energy consumption, short production cycle and few three wastes (waste gas, wastewater and industrial residue), namely a method for preparing m-nitrochlorobenzene, p-nitroanisole and o-nitroanisole from m-nitrochlorobenzene oil under an anhydrous system by a high pressure method. To achieve the above purpose, the technical scheme of the invention is as follows: m-nitrochlorobenzene oil is added into an autoclave, and methanol and sodium hydroxide are respectively added, wherein the mole ratio of sodium hydroxide to m-nitrochlorobenzene oil is 0.01-2.00:1, the mole ratio of methanol to m-nitrochlorobenzene oil is 1-20:1, reaction temperature is 10-200 DEG C, reaction time is 1-20 h, and pressure is 0.1-4.0 MPa; and products obtained after the reaction undergo gas chromatography, and content changes with the composition change of the reaction raw material m-nitrochlorobenzene oil. In comparison with traditional technologies, the method provided by the invention has advantages of low production cost, short process, low energy consumption and little pollution.
Owner:CHINA PETROLEUM & CHEM CORP +1
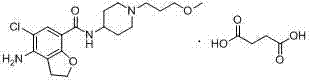
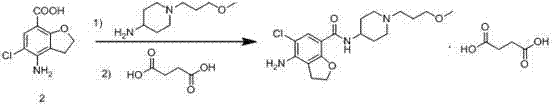
Method for preparing prucalopride
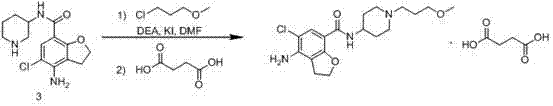
The invention belongs to the field of medicinal chemistry, and in particular relates to a preparation method of prucalopride. Prucalopride succinate is a 5-HT4 receptor stimulant with high selectivity and specificity, and is a novel intestinal motility drug. The drug has the characteristics of high selectivity, rapid onset and less untoward effect, and has wide clinical application prospect in the field of constipation treatment. The invention provides a new method for compounding prucalopride, wherein 4-nitro-5-chlor-2,3-dihydrobenzofuran-7-methanoic acid and 1-(3-methoxypropyl)-4-piperidinamine are employed as initial materials to prepare prucalopride; and the preparation method is simple and convenient to operate, less in side reaction, high in yield, mild in reaction condition and convenient for large-scale industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
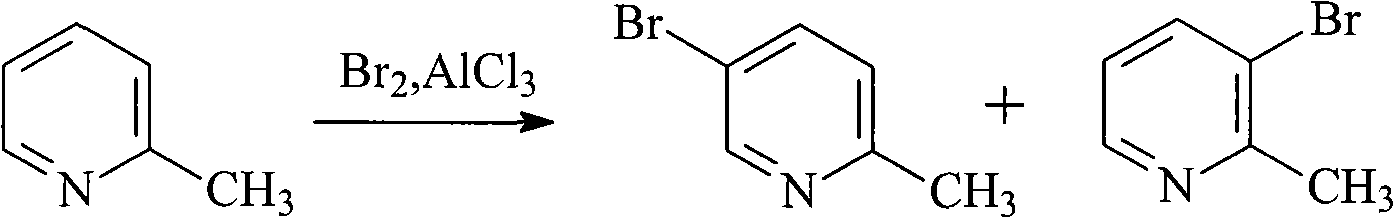
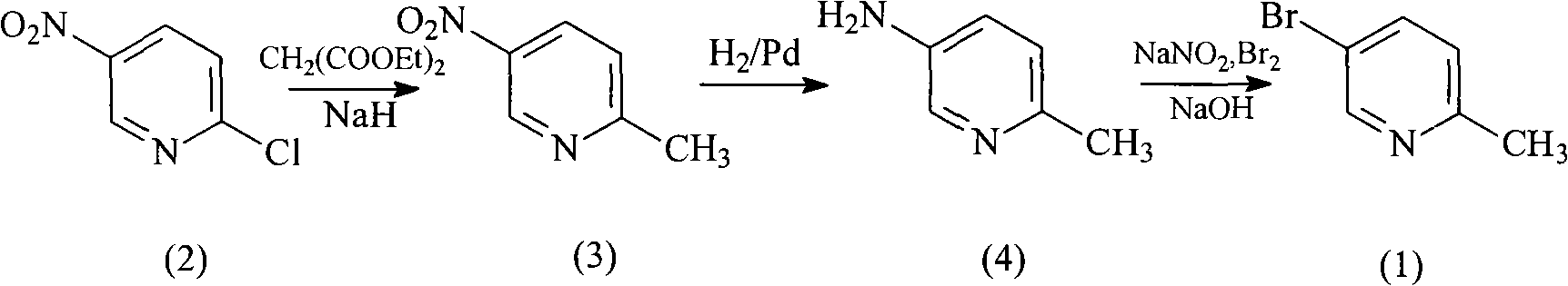
Method for preparing 5-bromo-2-methylpyridine
InactiveCN101560183AHigh yieldLow priceOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsCatalytic effectSodium nitrite
The invention discloses a method for preparing intermediate 5-bromo-2-methylpyridine. In the prior art, the dosage of aluminium trichloride is large; the catalytic effect is poor; the by-products are more; the yield of products is low; and the obtained products are difficult to separate. The method comprises the following steps: reacting diethyl malonate with alkali metal to generate salts, dripping 5-nitryl-2-chloropyridine into the salts for condensation reaction, and subsequently performing decarboxylation on the obtained product under acidic condition to obtain 5-nitryl-2-methylpyridine; performing hydrogenation reduction on the 5-nitryl-2-methylpyridine under the catalysis of Pd / C catalyst to obtain 5-amido-2-methylpyridine; and reacting the 5-amido-2-methylpyridine with acid to generate salts, dripping bromine, dripping a sodium nitrite water solution, and obtaining the 5-bromo-2-methylpyridine. The method has mild reaction conditions, easy operation, simple post-treatment, good catalytic effect, high yield of each step and high yield of final products, and is particularly suitable for industrialized production.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
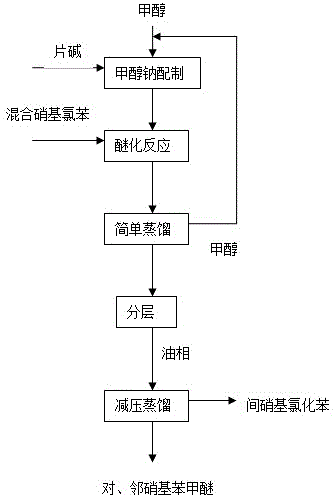
Method for reducing generation of nitrophenol in preparation process of nitroanisole
InactiveCN105566121AHigh selectivityHigh yieldOrganic chemistryOrganic compound preparationSodium methoxideNitrobenzene
A special feeding mode is adopted in the invention to reduce generation of nitrophenol, and is characterized in that a sodium methoxide solution prepared from methanol and sodium hydroxide is added to an autoclave in t he production process of nitroanisole from m-oil (o-nitrochlorobenzene, p-nitrochlorobenzene and m-nitrochlorobenzene) in the autoclave, so generation of nitrophenol is reduced, thereby the reaction time is greatly shortened, the raw material conversion rate is improved, the reaction selectivity and the yield of the above product are improved, the cost is reduced, and three wastes are reduced.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing 3,3'-dichlorobenzidine hydrochloride through rearrangement
InactiveCN104610072ALow viscosityEnhanced mass transferPreparation by rearrangement reactionsPtru catalystRearrangement reaction
The invention discloses a method for preparing 3,3'-dichlorobenzidine hydrochloride through the rearrangement reaction of hydrogen chloride. By using an existing technique, ortho-nitrochlorobenzene is added into an organic solvent and alkaline liquor under the catalysis of a precious metal catalyst and a cocatalyst, and then the obtained object carries out a coupled reduction reaction with hydrogen, so that 2,2'-dichlorohydrazobenzene is obtained; and hydrogen chloride gas is fed into a 2,2'-dichlorohydrazobenzene solution dissolved by using an organic solvent to carry out a rearrangement reaction, so that 3,3'-dichlorobenzidine hydrochloride is obtained. The yield can reach over 95%, the reaction process can be intermittent, semi-continuous and continuous reaction processes, and the method is low in viscosity of reaction process, easy to uniformly stir and low in energy consumption, and has a yield higher than that in a traditional process, therefore, the method has a good industrialization prospect.
Owner:NINGXIA LANFENG FINE CHEM CO LTD
O-chloroaniline preparation method
InactiveCN110655470AHigh purityInhibition of mass transferAmino compound purification/separationOrganic compound preparationPtru catalystFluid phase
The invention provides ano-chloroaniline preparation method, wherein the raw materials comprise: o-nitrochlorobenzene, a catalyst, dicyandiamide, a solvent, nitrogen and hydrogen. According to the invention, Ru / C is used as the catalyst, and has characteristics of simple components, stable activity and high selectivity, and dicyandiamide is used as the dechlorination inhibitor, so that the dechlorination side reaction is effectively inhibited, the conversion rate is high, and the selectivity is high; by improving the structure of the rectifying tower, the vapor-liquid mass transfer performancein heat transfer and mass transfer is improved, and the contact area between the gas phase and the liquid phases is increased by utilizing the auxiliary member; the turbulent flow degree is effectively increased through the inflow weir having the step structure and the turbulent flowblock, so that the mass transfer and heat exchange efficiency and the mass transfer and heat exchangerate between the gas phase and the liquid phases are improved; and the treatment and circulation capacities of the liquid phase are improved through the circulation tank, and the gas-liquid phase contact time is favorably prolonged by using the baffle arrangement of the downcomer and the inner diameter change, so that the yield and the purity of o-chloroaniline are improved.
Owner:滨海县星光化工有限公司
Method for preparing 3, 4'-diaminodiphenyl ether
PendingCN111072503ALess Process WastewaterShort process stepsOrganic compound preparationAmino-hyroxy compound preparationM-aminophenolDiaminodiphenyl ether
The invention discloses a method for preparing 3, 4'-diaminodiphenyl ether, which mainly comprises the following steps: adding m-aminophenol, an acid-binding agent and a solvent into a condensation reaction kettle, stirring, heating under the protection of inert gas, keeping the temperature at 100-120 DEG C, dropwisely adding p-nitrochlorobenzene, keeping the temperature for 2-4 hours after 5-7 hours, and continuing the reaction; after the reaction is finished, filtering to remove inorganic salt, transferring a filtrate into a hydrogenation kettle, adding a noble metal catalyst and a cocatalyst, performing nitrogen replacement, maintaining the temperature at 60-80 DEG C, and introducing hydrogen for catalytic hydrogenation under the hydrogen pressure of not more than 0.5 MPa; after the reaction is finished, filtering out the catalyst, distilling the filtrate, removing the solvent, rectifying, collecting 206-210 DEG C (2-3 mmHg) fraction which is white 3, 4'-diaminodiphenyl ether, and packaging under the protection of nitrogen. The method is short in process step, low in energy consumption and low in production cost, the content of the obtained 3, 4'-diaminodiphenyl ether is largerthan 99.5%, and the total molar yield is larger than 93%.
Owner:南通汇顺化工有限公司
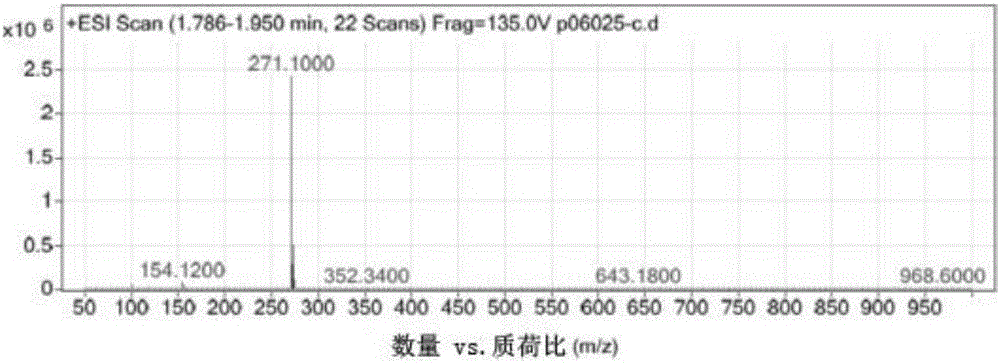
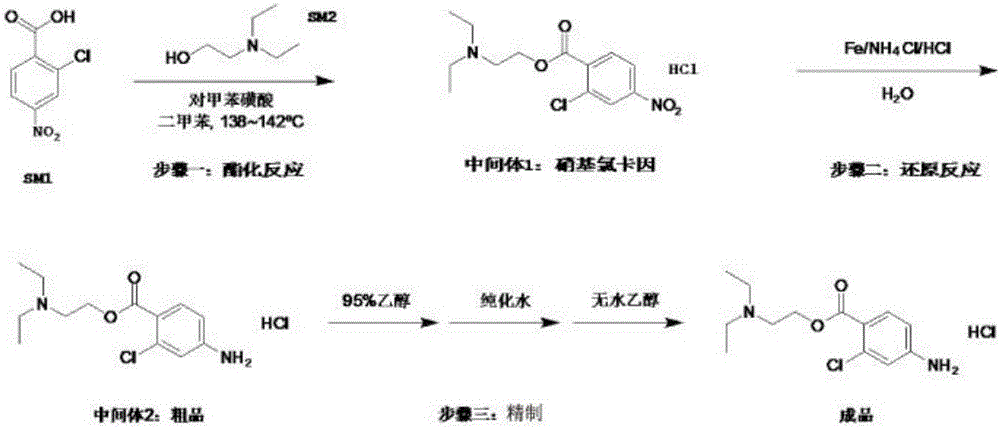
Preparation method of chloroprocaine hydrochloride
InactiveCN105968019AIncrease reaction rateHigh reaction yieldOrganic compound preparationAmino-carboxyl compound preparation2-chloro-4-nitrobenzoic acidPara-toluenesulfonic acid
The invention belongs to the technical field of organic drug synthesis and particularly relates to a preparation method of chloroprocaine. The preparation method includes the steps that 1, 2-chloro-4-nitrobenzoic acid and 2-diethylaminoethanol reaction raw materials are added into a xylene reaction solvent, para-toluenesulfonic acid or immobilized liquid acid or faintly acid metal salt is added to serve as a catalyst, heating is conducted till reflux is achieved, a reaction is carried out for a certain period of time, aftertreatment is carried out, and a water solution of nitrochlor cain is obtained; 2, ammonium chloride and iron powder are added into the water solution of nitrochlor cain, a heating reaction is carried out, aftertreatment is carried out, and crude chloroprocaine hydrochloride is obtained; refining is carried out, and chloroprocaine hydrochloride is obtained. The direct esterification reaction time is short, the production period is shortened by 30% or above, the total yield of the finished product is larger than 30%, and energy consumption is reduced by 25% or above. The content of chloroprocaine hydrochloride is not smaller than 99.0%, the content of related substances is not larger than 1.0%, and the content of a residual solvent (dimethylbenzene) does not exceed 0.1%. The preparation method is suitable for industrial production.
Owner:WUXI KAIFU PHARMA
2-arylmethylthio-6-(tetrahydroquinoline-1-methyl)-4-pyrimidinone derivatives and their preparation methods and applications
InactiveCN102285967AImprove hydrophobicityEnhanced hydrogen bondingOrganic active ingredientsOrganic chemistryBrominePyrimidine
The present invention provides a 2-[(substituted phenylamino)carbonylmethylthio]-6-(2,6-dichlorobenzyl)-3H-pyrimidin-4-one derivative, the general structural formula I is as follows: Where R1 is: H, methyl or ethyl; R2 is: 2-bromoacetophenone, p-methyl-2-chloroacetophenone, p-chloro-2-chloroacetophenone, p-nitro- 2-chloroacetophenone, p-cyano-2-chloroacetophenone, p-methoxy-2-chloroacetophenone, p-fluoro-2-chloroacetophenone, benzyl bromide, p-methyl Benzyl chloride, p-chlorobenzyl chloride, p-nitrobenzyl chloride, p-cyanobenzyl chloride, p-methoxybenzyl chloride or p-fluorobenzyl chloride. The invention also relates to a preparation method of the compound and its application as an HIV inhibitor.
Owner:SHANDONG UNIV
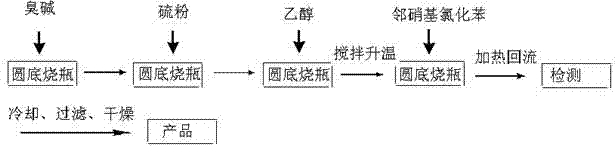
Rubber peptizer intermediate 2,2'-dinitro-diphenyl disulfide and preparation method thereof
InactiveCN102558000AImprove responseHigh purityHydropoly/poly sulfide preparationPolymer scienceVulcanization
The invention provides diphenyl disulfide type vulcanized peptizer intermediate, namely 2,2'-dinitro-diphenyl disulfide and a preparation method thereof. The preparation method is characterized in that ortho-nitrochlorobenzene, industrial sodium sulfide and sulfur powder are adopted as raw materials, and the 2,2'-dinitro-diphenyl disulfide is synthesized in ethanol. The preparation method has the advantages of simple reaction process and high purity of obtained products.
Owner:LIAOCHENG UNIV
Application of antioxidant in distillation reaction of p-chloroaniline
ActiveCN103467302AImprove stabilityImprove heat resistanceAmino compound purification/separationP-chloroanilineHydrogen
The invention relates to an application of an antioxidant in distillation reaction of p-chloroaniline. The process comprises the following steps: simultaneously adding a generated crude product, namely the p-chloroaniline and the antioxidant into a distillation kettle after the reaction of p-nitrochlorobenzene and hydrogen is completed and reacting during the distillation process to get a final product, namely the p-chloroaniline. The antioxidant comprises the component of sodium sulfide, and the concentration is 0.1%. The temperature of the distillation reaction is 130 DEG C, and the pH value during the reaction process is 10-11. The application disclosed by the invention has the advantages that compared with other antioxidants, the application of the antioxidant has better stability, good heat resistance and good oxidation resistance, and can keep the color and the appearance of the material for a long time. The application disclosed by the invention further has the advantages of simple process, low production cost, low toxicity and good oxidation resistance of the product, and is suitable for green industrial production.
Owner:HULUDAO TIANQI SHENGYE CHEM
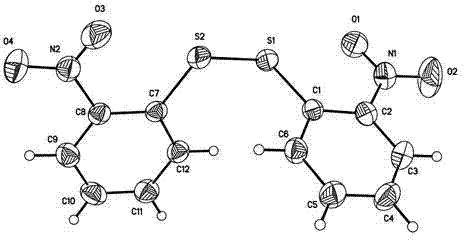
2-chloro-10,11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method
InactiveCN103524455ASimple methodMild reaction conditionsOrganic chemistryO-nitrochlorobenzeneThiazepine
The invention discloses a 2-chloro-10, 11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method. The method comprises the steps that o-nitro chloro benzene which is low in price and easy to get is used for condensation with 4-chlorothiophenol to obtain 2-nitro-4'-chlorodiphenyl sulfide, then reduction is carried out to obtain 2-amino-4'-chlorine-diphenyl sulfide; then acylation with chloroformate is carried out, and finally Friedel-Crafts reaction is carried out to obtain 2-chloro-10, 11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one. According to the preparing method, reaction conditions are mild, raw materials used for the reaction are wide in source, reaction yield is high, reproducibility is good, product quality is stable, the preparing process is simple, initial raw material o-nitro chloro benzene is large-tonnage products in China, the o-nitro chloro benzene is low in price and easy to get, production cost is greatly lowered, and industrial production is convenient.
Owner:SUZHOU JINGYE MEDICINE & CHEM
Dual-response dipeptide supramolecular polymer, and preparation method and application thereof
ActiveCN113683661AEasy to makeHigh fluorescence intensityOrganic active ingredientsNanomedicineNitroimidazolePolymer science
The invention belongs to the technical field of high-molecular compounds. The invention provides a dual-response dipeptide supramolecular polymer, which is a dual-sensitive supramolecular polymer prepared by the following steps of: modifying a hypoxic response element p-nitrochloroformic acid benzyl ester or 2-(2-nitroimidazole-1-yl) acetic acid at an N terminal and modifying a temperature-sensitive element dendritic alkoxy ether at a C terminal through tryptophan glycine dipeptide by adopting a liquid phase synthesis method, and performing self-assembly in an aqueous solution. The supramolecular polymer is simple to prepare; the fluorescence intensity is greatly enhanced under the hypoxic condition through the fluorescence characteristic of tryptophan; meanwhile, the polymer can coat the medicine; the polymer is disassembled under the hypoxic condition to realize the release of the medicine; and a research basis is provided for the research and development of a novel nano-medicine carrier.
Owner:JINING MEDICAL UNIV
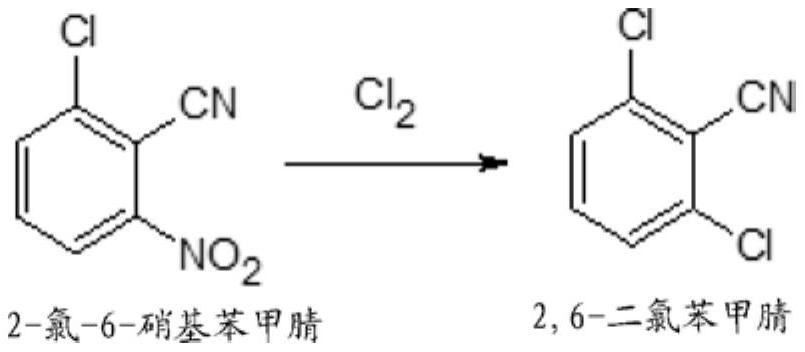
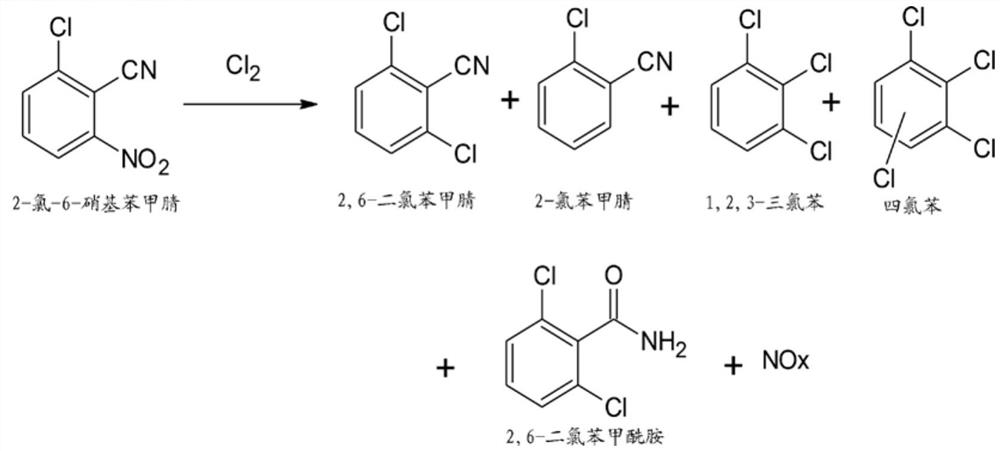
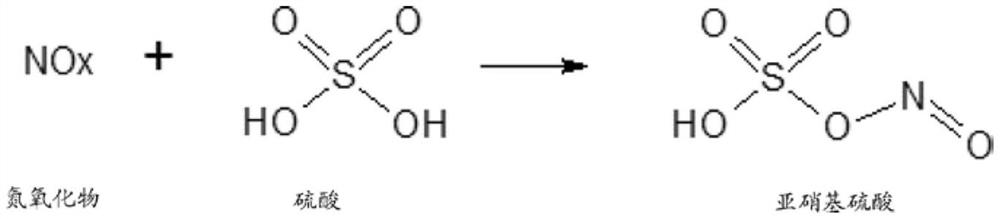
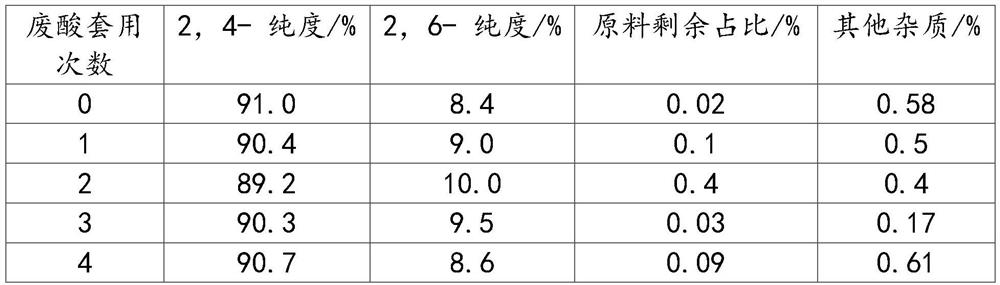
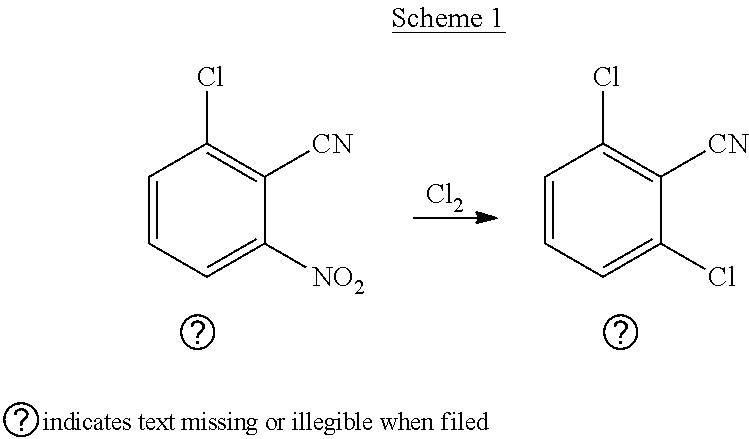
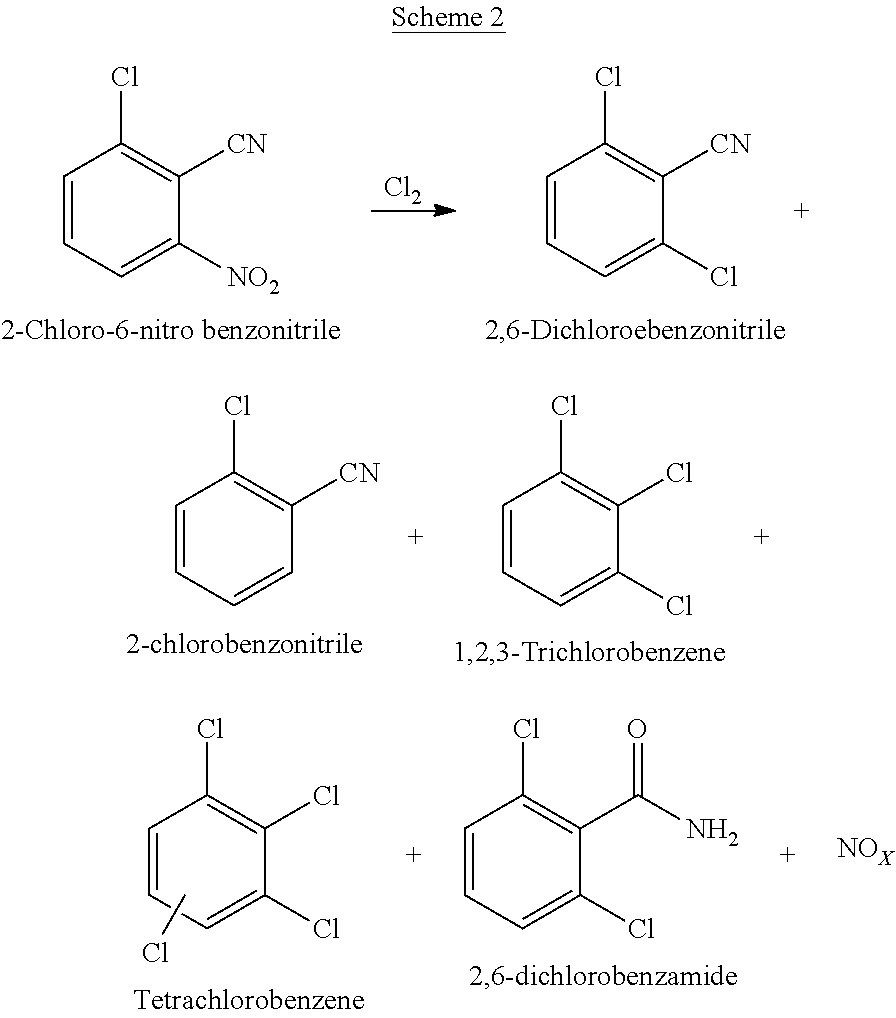
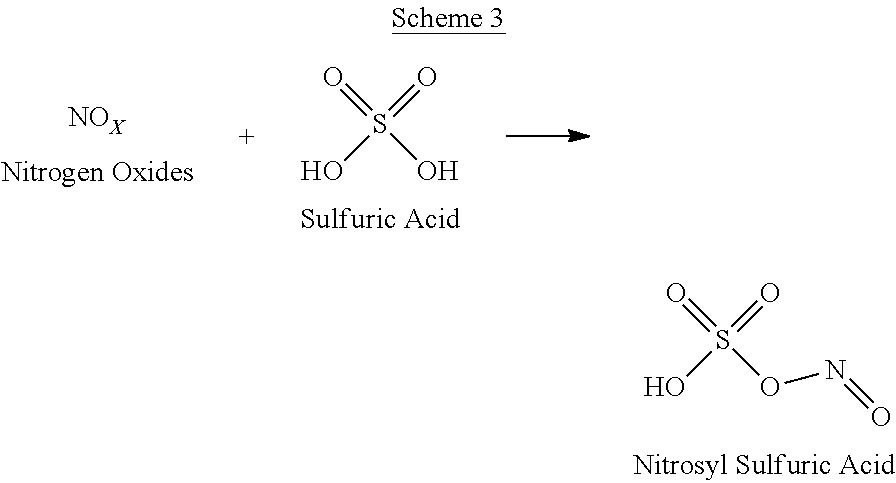
Process for preparation of 2,6-dichlorobenzonitrile
Disclosed herein a process preparation of 2,6-dichlorobenzonitrile. A process of making high yield, high purity 2,6-dichlorobenzonitrile including the selective de-nitrochlorination of 2-chloro-6-nitrobenzonitrile by treatment of the 2-chloro-6-nitrobenzonitrile with chlorine gas.
Owner:ARYSTA LIFESCIENCE CORP
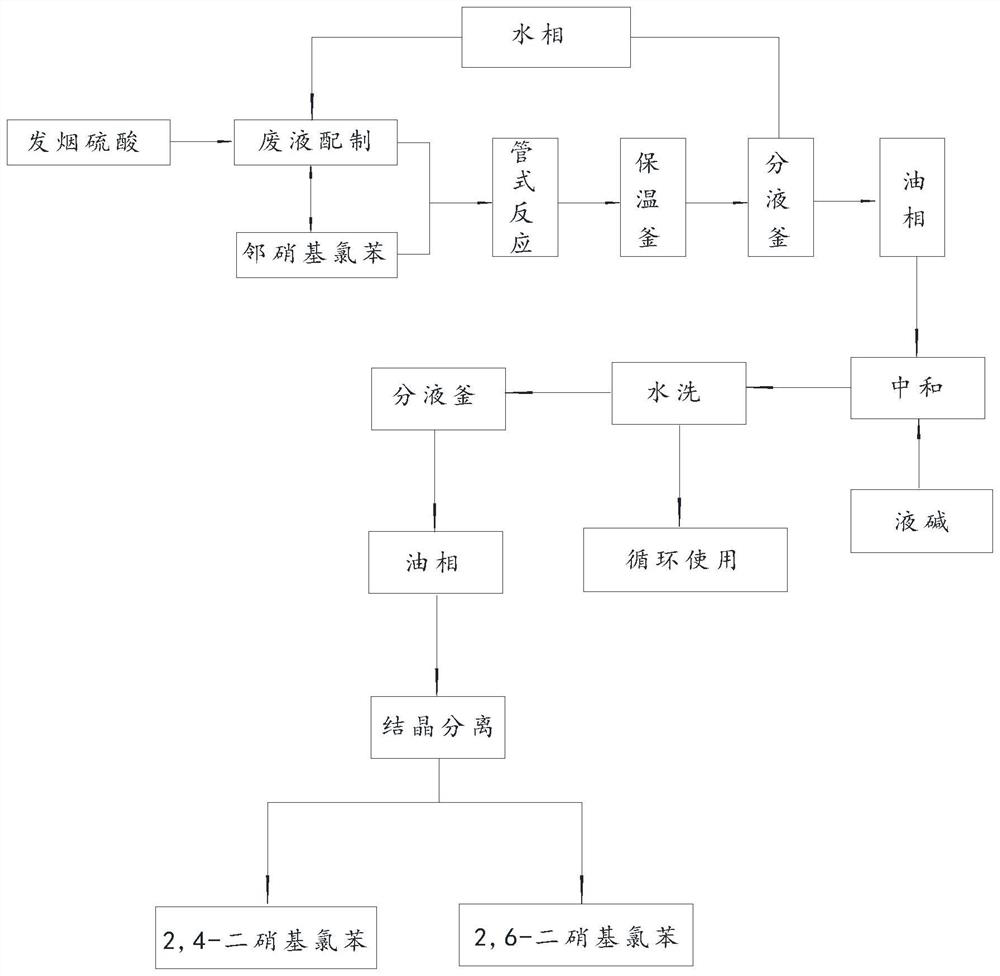
Clean production method of 2, 4-dinitrochlorobenzene
InactiveCN113354541AEmission reductionReduce manufacturing costOrganic compound preparationNitro compound preparationOleumNitration
The invention provides a clean production method of 2, 4-dinitrochlorobenzene, which comprises the following steps: by taking o-nitrochlorobenzene with the mass fraction of 99% as a raw material and a mixed acid solution prepared from sulfuric acid and nitric acid with the mass fraction of 98% as a nitrating agent, carrying out a continuous nitration reaction in a tubular reactor, pumping the nitrated mixed solution into a heat preservation kettle, after keeping the temperature for 2 hours, pumping the nitration mixed solution into a liquid separation kettle, and separating an upper-layer oil phase, namely a mixed solution of 2, 4-dinitrochlorobenzene and 2, 6-dinitrochlorobenzene, and a lower-layer water phase, namely a waste acid solution; adding fuming sulfuric acid into the separated waste acid solution, so that the moisture content in the waste acid solution is 2% or below; neutralizing the separated mixed solution of 2, 4-dinitrochlorobenzene and 2, 6-dinitrochlorobenzene, washing with water and performing crystallization separation, and obtaining the main product 2, 4-dinitrochlorobenzene and the by-product 2, 6-dinitrochlorobenzene. The continuous production process is adopted, the cost is reduced, prepared waste acid is mechanically applied, waste acid emission is reduced, and the environmental burden is relieved.
Owner:天津泰研科技发展有限公司
Process for preparation of 2,6-dichlorobenzonitrile
ActiveUS20200157043A1Simple and industrially viableHigh yieldOrganic compound preparationPreparation by cyanide reactionPhysical chemistryNitrobenzene
Disclosed herein a process preparation of 2,6-dichlorobenzonitrile. A process of making high yield, high purity 2,6-dichlorobenzonitrile including the selective de-nitrochlorination of 2-chloro-6-nitrohenzonitrile by treatment of the 2-chloro-6-nitrobenzonitrile with chlorine gas.
Owner:ARYSTA LIFESCIENCE INC
Organic aggregate materials with naked eye visible color change after fluorescence enhancement
The invention relates to a type of organic aggregate materials with naked eye visible color change after fluorescence enhancement. The general chemical formula of the materials is described in the description, wherein R in the structural formula is hydrogen, oxygen methyl, N,N-dimethyl, N,N-diethyl, cyano, nitro, chloro or the like. Organic aggregates are obtained by adding organic molecules into a mixed solvent of a benign solvent (such as N,N-dimethyl formamide) and a non-benign solvent (such as water) in a solvent inducing way; the color change of the obtained materials are drastic naked eye visible after fluorescence enhancement.
Owner:CHONGQING UNIV
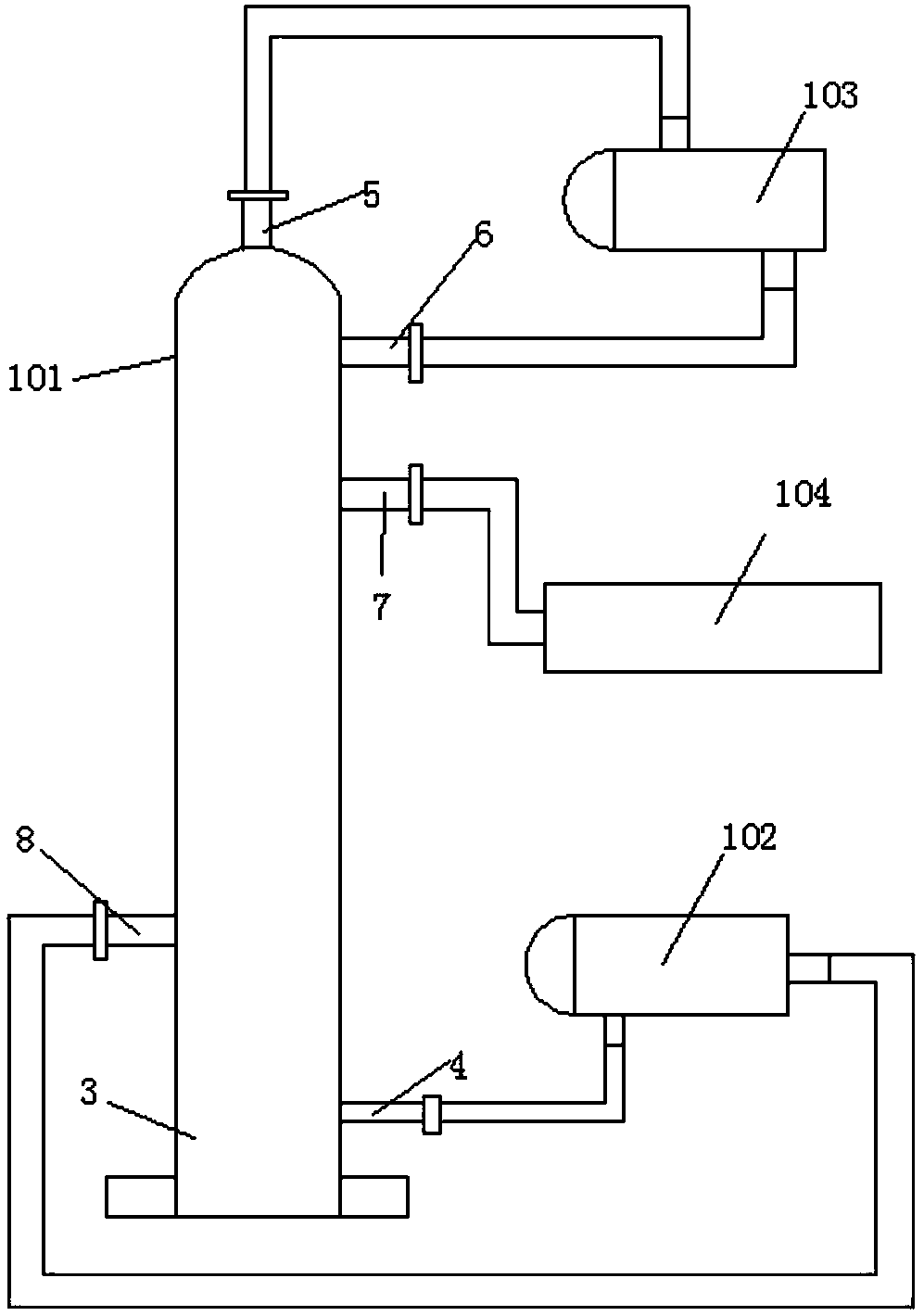
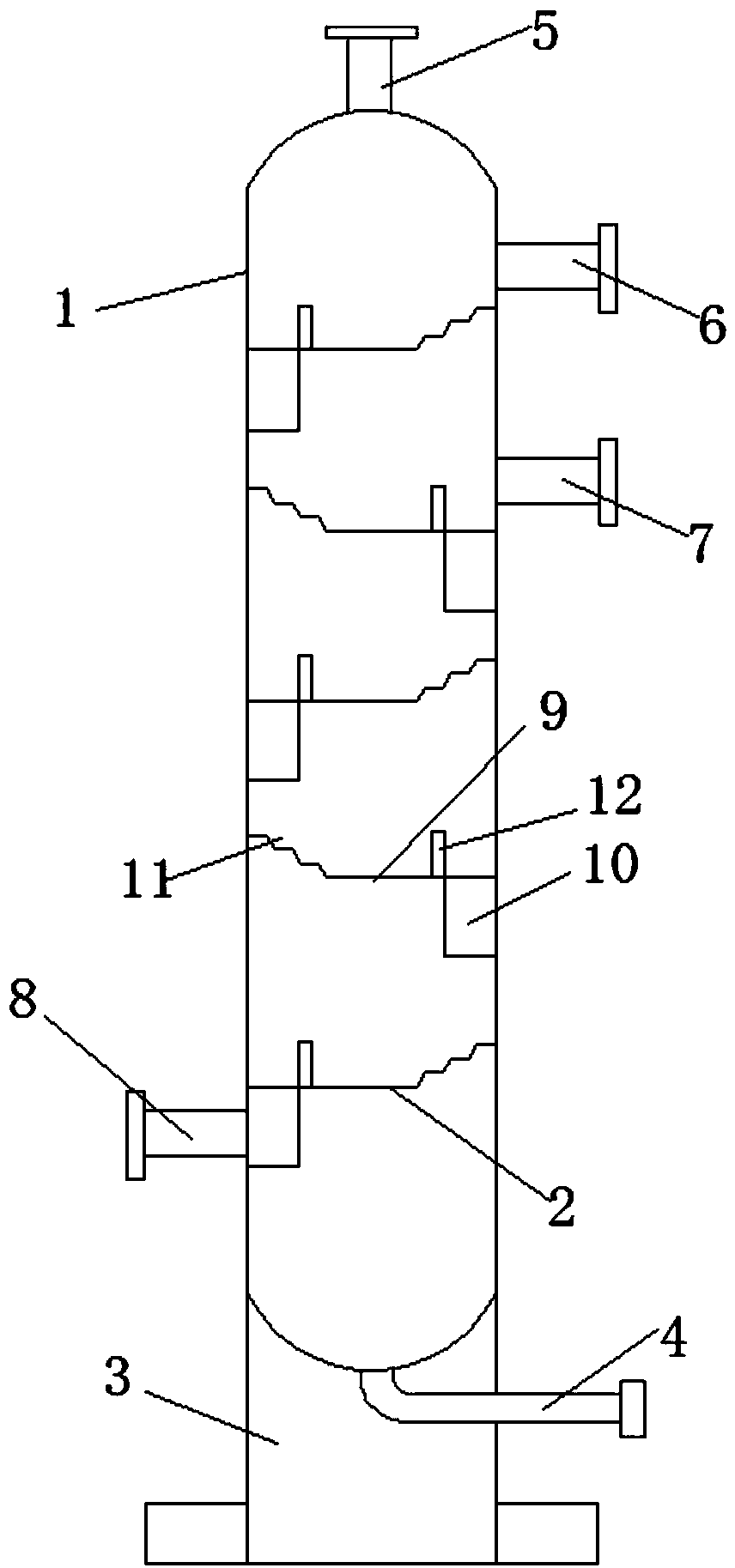
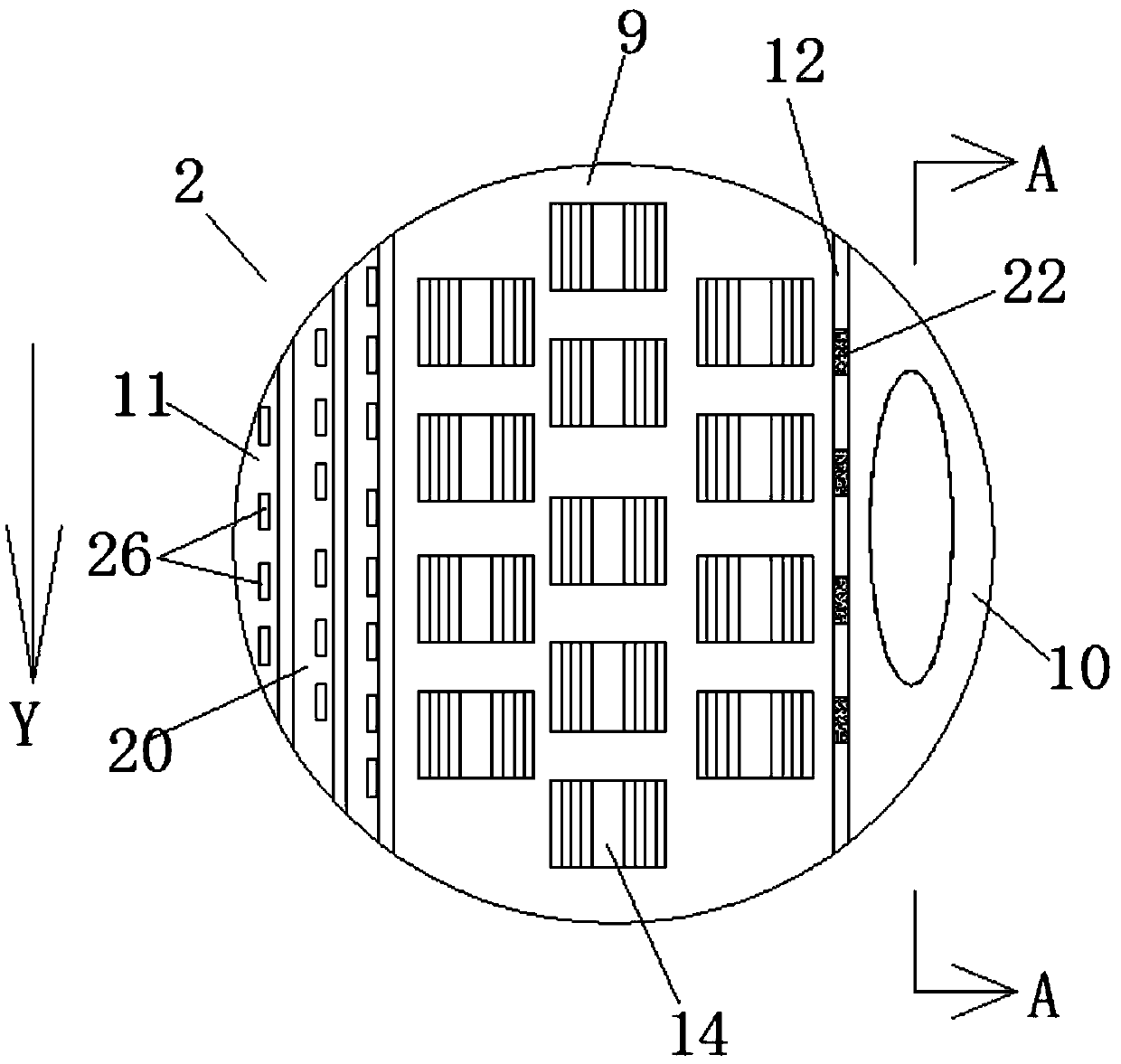
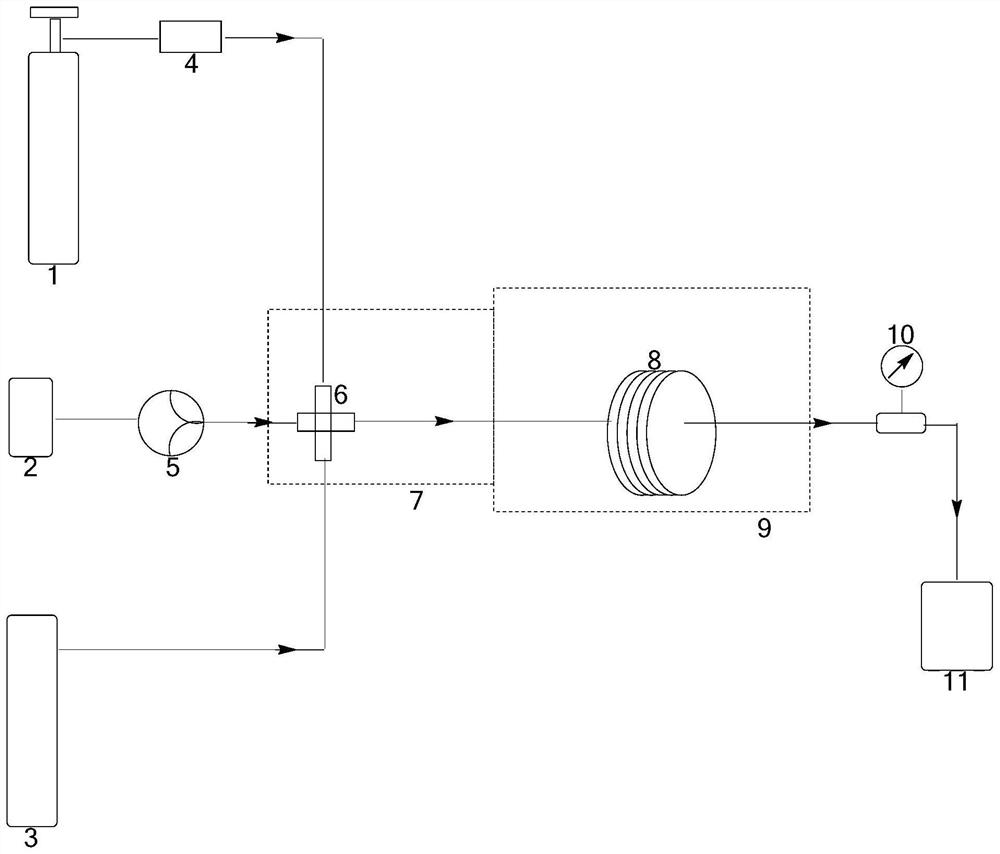
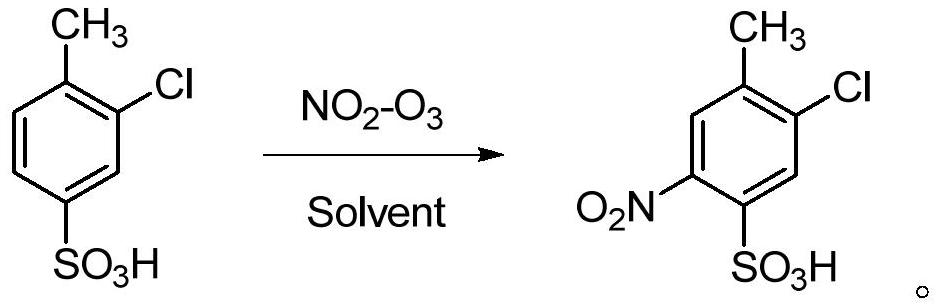
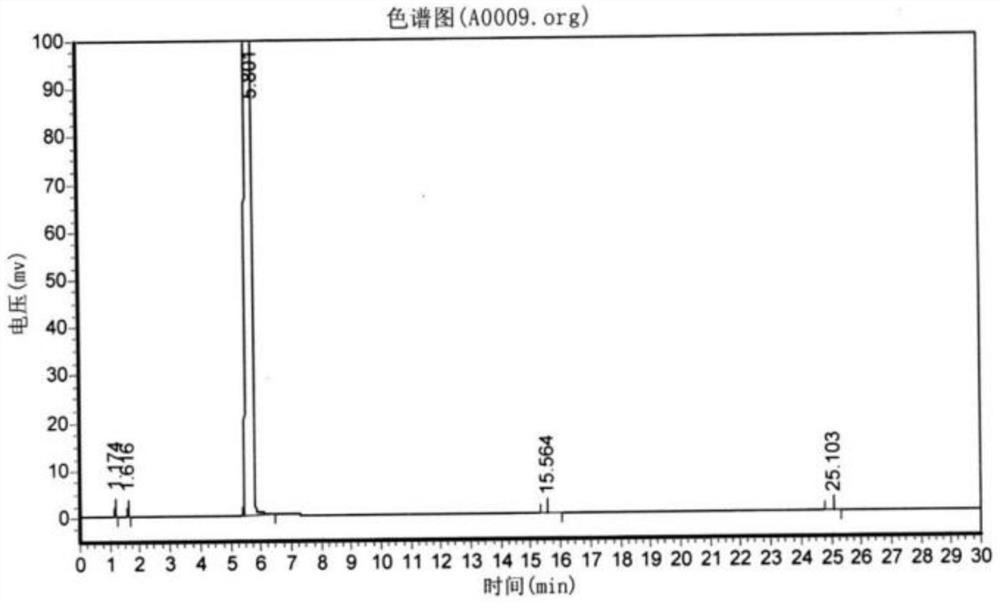
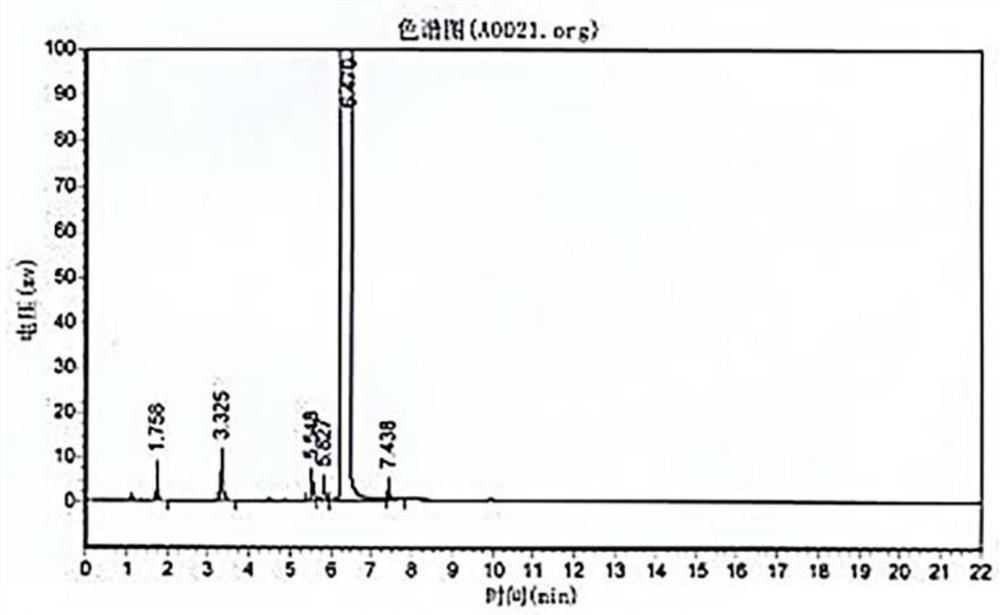
Method and device for pipelined preparation of 2-nitro-5-chloro-4-methylbenzenesulfonic acid
ActiveCN109180539BEasy to operateSimple post-processingSulfonic acid preparationPtru catalystNitration
The invention discloses a pipeline method and device for preparing 2-nitro-5-chloro-4-toluenesulfonic acid. The device comprises a nitrogen dioxide steel cylinder, a storage tank, an ozone generator,a flow pump, a gas flowmeter, a cross mixed joint, a reaction pipeline passing through an alumina interior coating, a cooling system, a heating system, a back pressure valve and a receiving tank. Themethod comprises the following steps: turning on the cooling system and the heating system for preheating; turning on the ozone generator, setting ozone flow, and regulating the back pressure valve; setting the flow pump and the gas flowmeter; and mixing the raw materials, nitrogen dioxide and ozone via the cross mixed joint, enabling the mixture to enter the reaction pipeline passing through an alumina interior coating for carrying out a nitration reaction, and performing after-treatment on the reaction solution, thereby obtaining the 2-nitro-5-chloro-4-toluenesulfonic acid. According to themethod disclosed by the invention, the catalyst alumina is arranged in the pipeline in a coating form, the catalyst is uniformly arranged, the catalytic efficiency is high, the packing does not need to be removed, and the operation is simple and convenient.
Owner:ZHEJIANG UNIV OF TECH
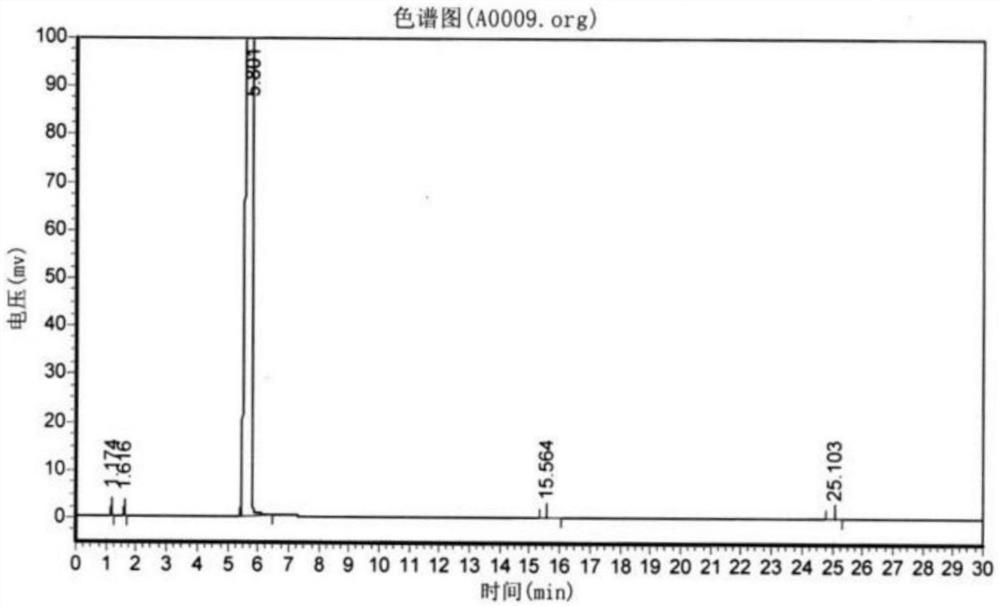
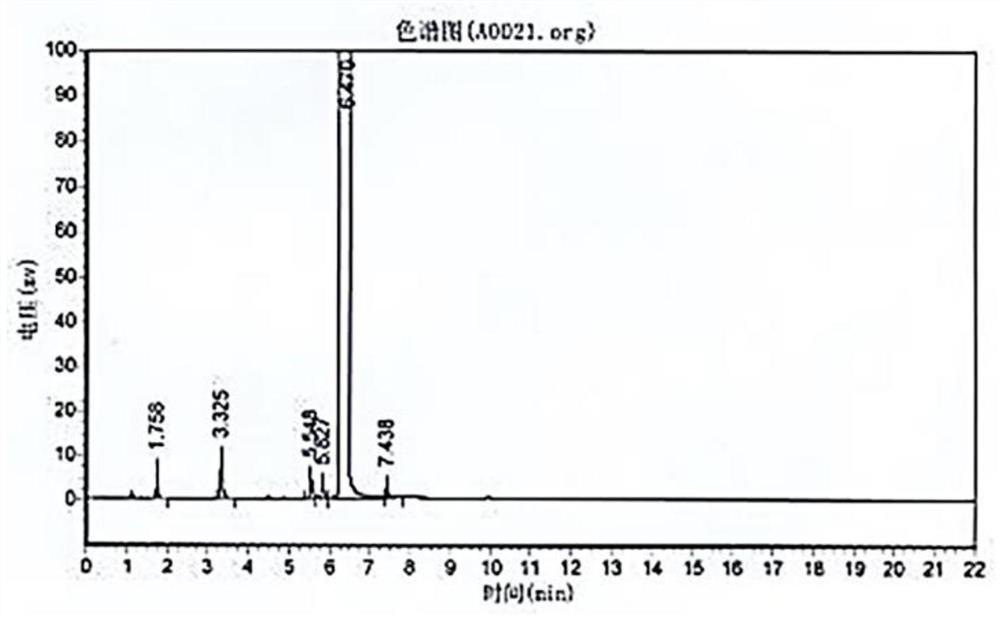
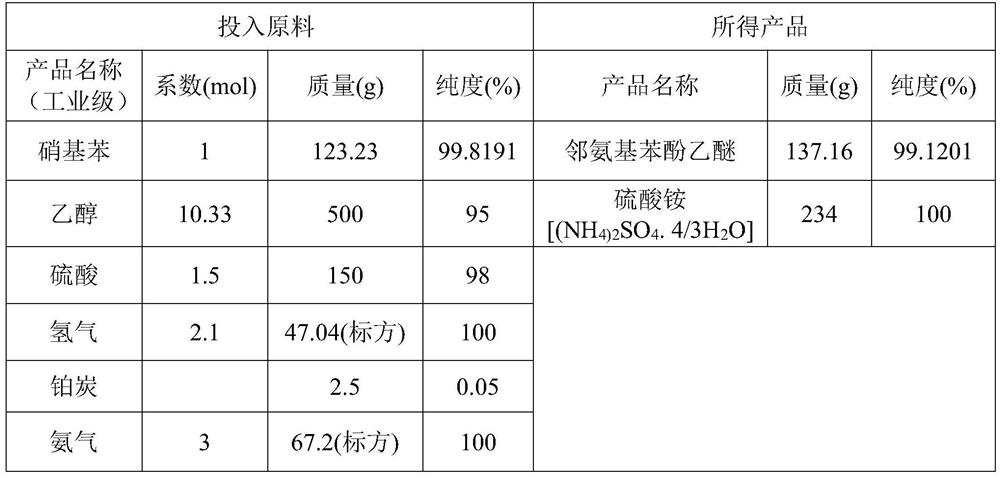
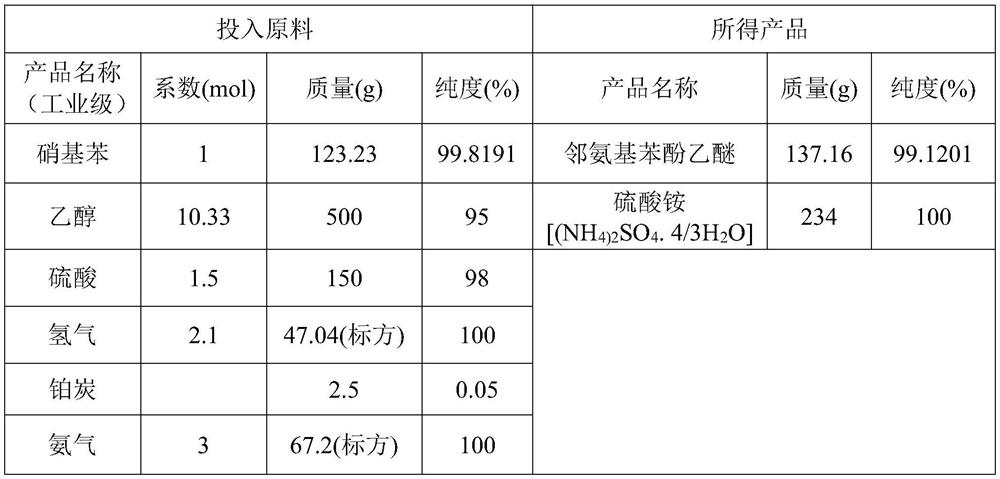
Preparation process of o-aminophenetole
PendingCN114478277ANo need for high temperature and high pressureImprove securityOrganic compound preparationAmino-hyroxy compound preparationNitrobenzeneDrug product
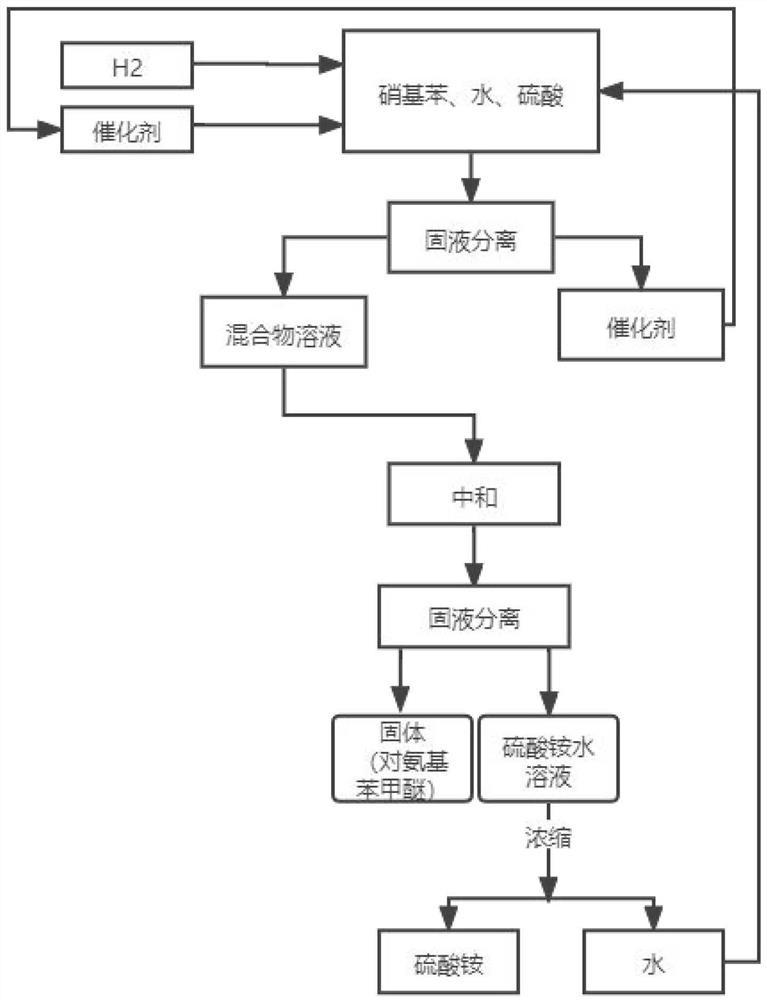
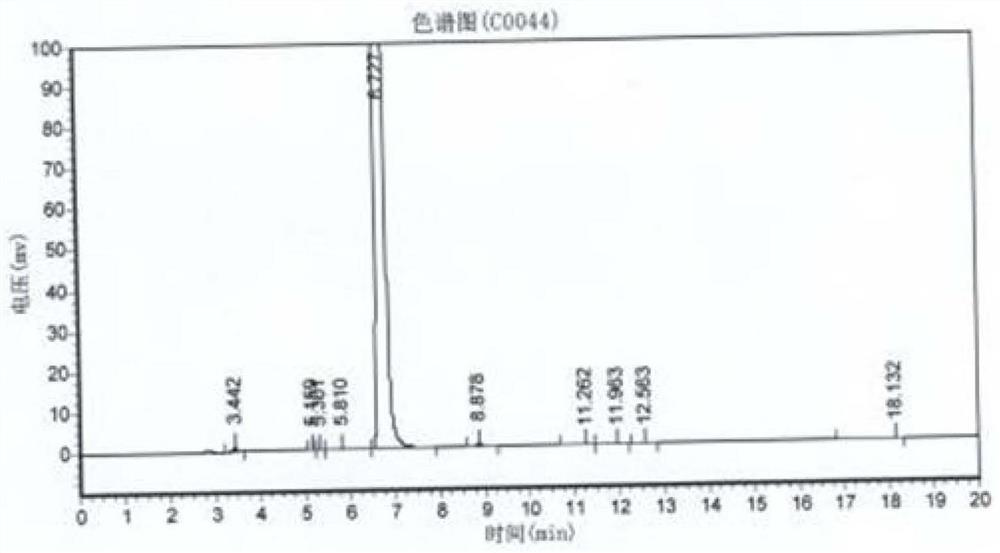
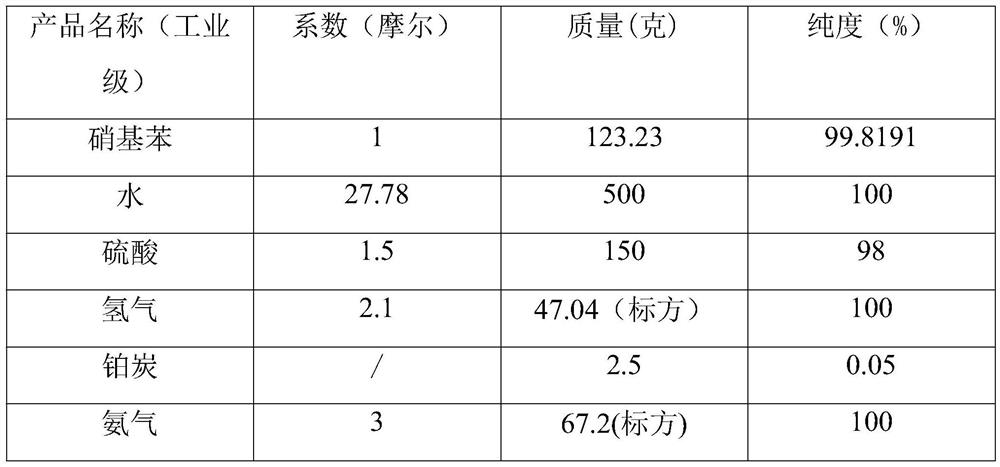
The invention relates to a preparation process of o-aminophenetole, which is characterized in that nitrobenzene, sulfuric acid and ethanol mixture are used as raw materials to prepare the o-aminophenetole, and the process is used for preparing the o-aminophenetole and ammonium sulfate through the steps of catalytic hydrogenation reduction, solid-liquid separation, water addition for dissolution, solid-liquid separation, acid-base neutralization, oil-water separation and the like. According to the method, nitrobenzene is adopted to replace o-nitrochlorobenzene, high temperature and high pressure are not needed, three wastes are not discharged, the method is a safe, environment-friendly and efficient o-aminophenetole synthesis process, and the purity of the obtained product reaches 99.90% or above and completely reaches the drug standard.
Owner:江苏普洛德化学科技有限公司
Preparation method of ortho-aminophenol
PendingCN114031509ANo need for high temperature and high pressure reductionImprove securityOrganic compound preparationAmino-hyroxy compound preparationM-aminophenolPtru catalyst
The invention provides a preparation method of ortho-aminophenol, which specifically comprises the following steps: (1) taking water, nitrobenzene and sulfuric acid as raw materials, adding a catalyst, and preparing an initial reaction solution under the condition of introducing hydrogen; (2) carrying out solid-liquid separation on the initial reaction solution, taking a solid substance as the catalyst, and reusing the solid substance in S1, wherein a liquid substance is a mixture solution; and (3) adjusting the pH of the mixture solution by a basic substance to neutrality. According to the preparation method of ortho-aminophenol, nitrobenzene is used for replacing ortho-nitrochlorobenzene, high-pressure hydrolysis and high-temperature and high-pressure reduction are not needed, reaction conditions are mild, the operation process is simple, safety is high, production efficiency is high, productivity is high, the product cost is reduced, and the overall benefit is increased.
Owner:江苏普洛德化学科技有限公司
A kind of synthetic method of 1-hydroxyl-pyrrolo[2,3-c]piperidine
The invention discloses a synthesis method of 1-hydroxy-pyrrolo[2,3-c]piperidine, which adopts the following steps: (1) 1-nitro-5-chloro- 1-pentene is the starting material, reacts with TosMIC shown in formula (Ⅴ) under alkaline conditions to obtain compound (VI); (2) compound (VI) generates compound (VII) through catalytic hydrogenation; (3) Compound (VII) generates compound (III) under the action of a base. The synthesis method of 1-hydroxy-pyrrolo[2,3-c]piperidine of the present invention reduces the process cost and production risk.
Owner:ZHEJIANG UNIV OF TECH
Production process of p-nitroaniline
ActiveCN108689859BWell mixedHigh yieldOrganic compound preparationChemical/physical/physico-chemical stationary reactorsSlider bearingP-Nitroaniline
Owner:苏州市罗森助剂有限公司
Novel method for synthesizing 3, 5-dinitrobenzyl chloride
InactiveCN111393300AReduce usagePromote reductionOrganic chemistryOrganic compound preparationPtru catalystBenzyl chloride
The invention provides a novel method for synthesizing 3, 5-dinitrobenzyl chloride. The novel method comprises the following steps: a, carrying out reduction reaction on 3, 5-dinitrobenzoyl chloride and a reducing agent in a first solvent to obtain 3, 5-dinitrobenzene methanol; and b, carrying out chlorination reaction on the 3, 5-dinitrobenzene methanol, a chlorination reagent and a catalyst in asecond solvent to obtain the 3, 5-dinitrobenzyl chloride. According to the method, the reduction process of the 3, 5-dinitrobenzoyl chloride is remarkably improved, a simple reducing agent is used, nitro reduction is avoided under the assistance of Lewis acid and Lewis alkali, and the reaction selectivity and the product yield are improved.
Owner:NANJING UNIV OF TECH
Preparation process of o-aminophenetole
InactiveCN113402403ANo need for high temperature and high pressureImprove securityOrganic compound preparationAmino-hyroxy compound preparationNitrobenzeneDrug product
The invention relates to a preparation process of o-aminophenetole. The is characterized in that nitrobenzene, sulfuric acid and ethanol mixture are used as raw materials to prepare the o-aminophenetole; and the process is used for preparing the o-aminophenetole and ammonium sulfate through the steps of catalytic hydrogenation reduction, solid-liquid separation, water addition for dissolution, solid-liquid separation, acid-base neutralization, oil-water separation and the like. According to the method, nitrobenzene is adopted to replace o-nitrochlorobenzene, high temperature and high pressure are not needed, three wastes are not discharged, and the method is a safe, environment-friendly and efficient o-aminophenetole synthesis process; and the purity of the obtained product reaches 99.90% or above and completely reaches the drug standard.
Owner:江苏普洛德化学科技有限公司
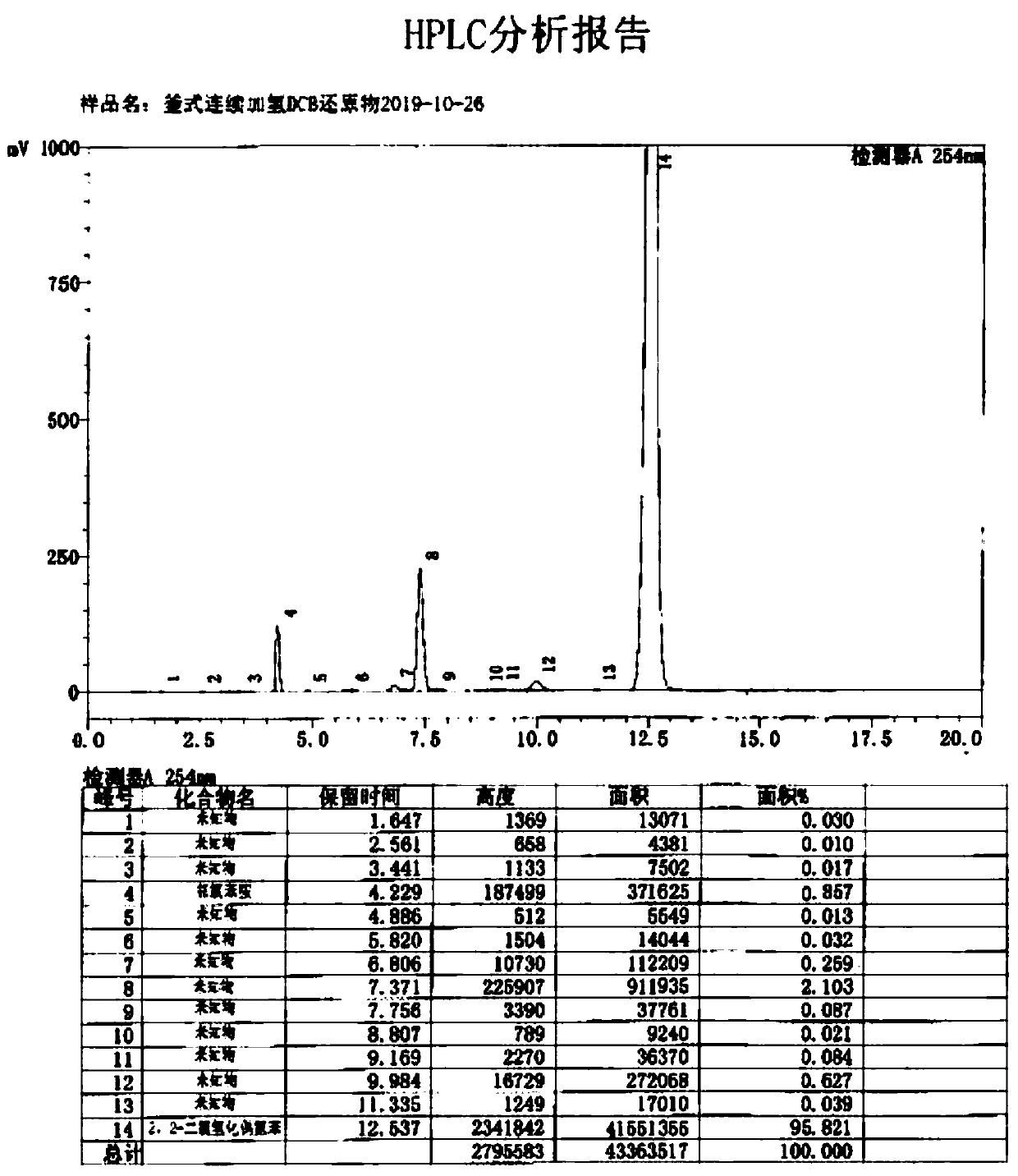
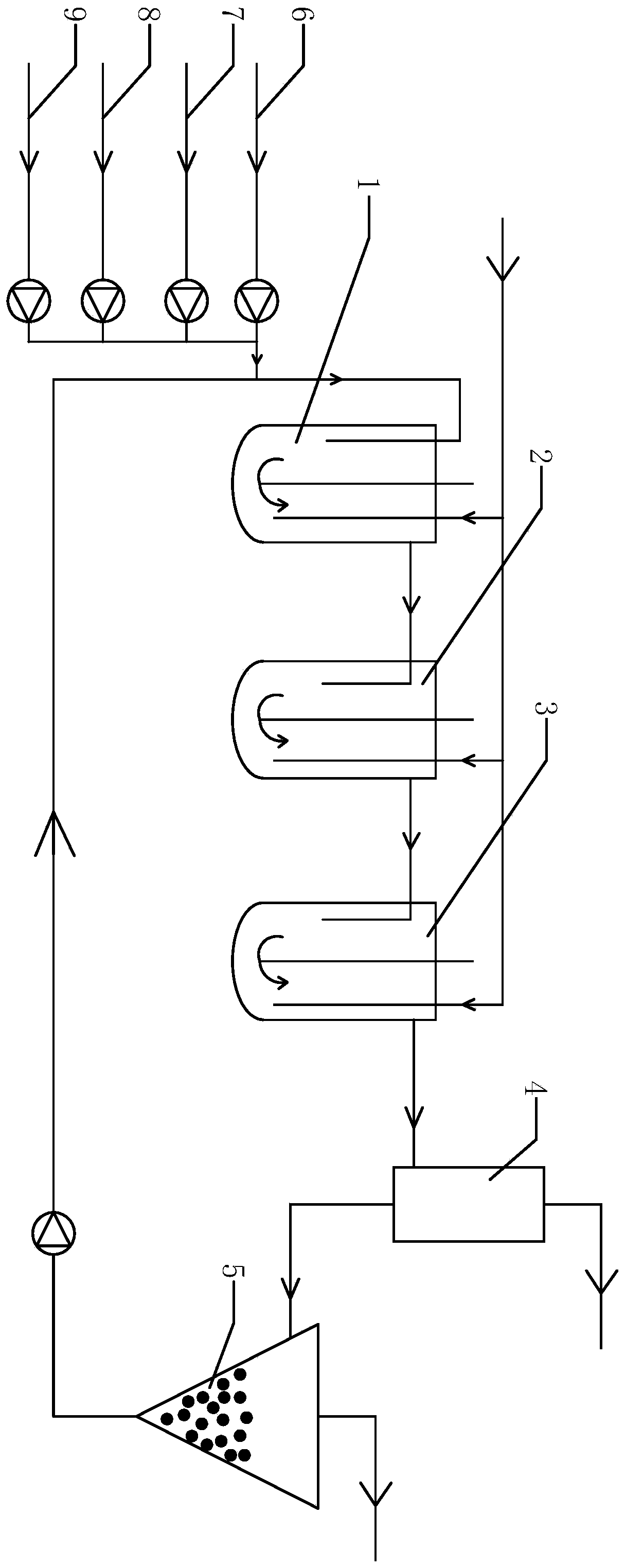
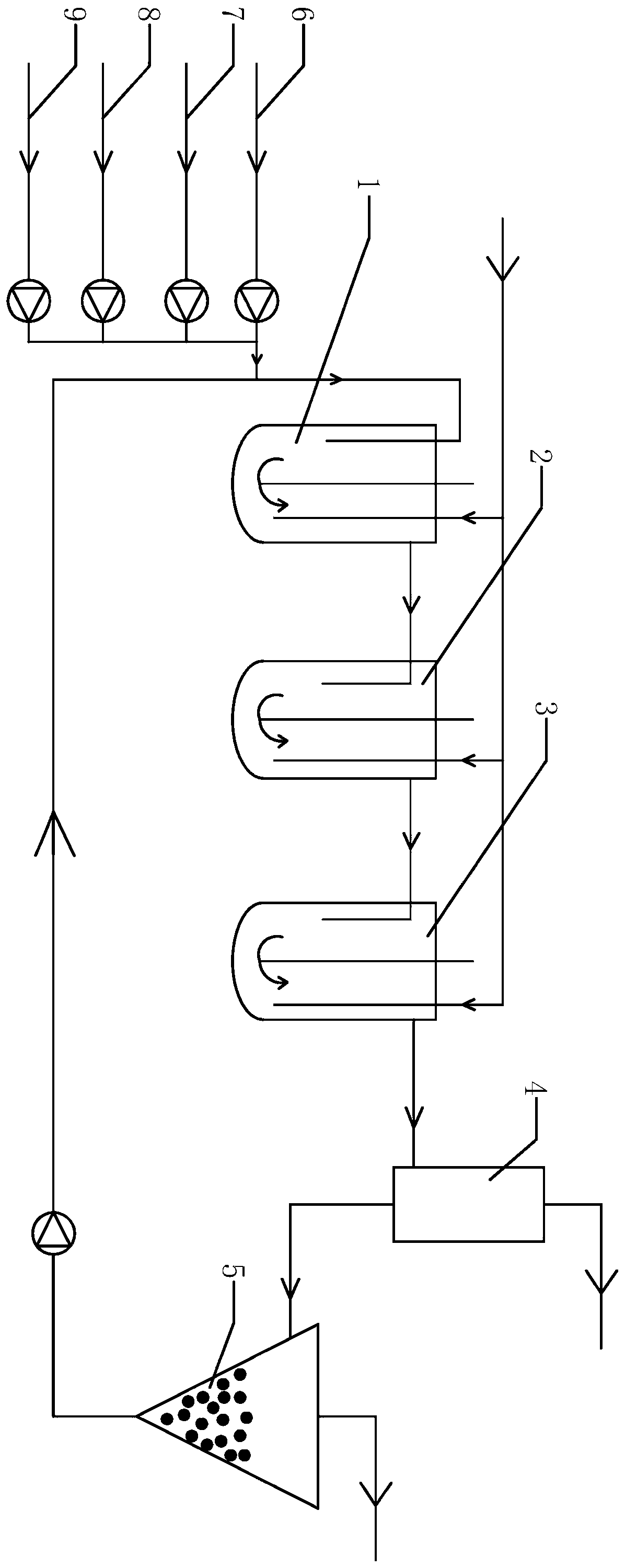
Method for preparing DCB reduzate through continuous catalytic hydrogenation reduction
ActiveCN111116407AReduce volumeImprove reaction stabilityHydrazine preparationChemical recyclingPtru catalystBatch reaction
The invention provides a method for preparing a DCB reduzate through continuous catalytic hydrogenation reduction. The method comprises the following steps: adding o-nitrochlorobenzene, toluene, caustic soda liquid with a concentration of 30%, desalted water and a Pt / C catalyst into three continuous tank reactors connected in series, and ending a first-stage intermittent reaction until all reactions do not absorb hydrogen; opening overflow discharge valves of the three tank reactors, and simultaneously pumping o-nitrochlorobenzene, methylbenzene, caustic soda liquid with the concentration of 30% and desalted water into the first tank reactor in proportion by using a metering pump; and continuously introducing hydrogen for catalytic hydrogenation to prepare the DCB reduzat, namely, 2,2-dichlorohydrazobenzene. According to the invention, the loss of the catalyst due to frequent filtering in a batch reaction process can be effectively reduced, internal recycling of the catalyst is achieved, and the service life of the catalyst is prolonged; meanwhile, the tank reactors are relatively small in size, reaction stability is good, product dechlorination is relatively low, selectivity is high and relatively few byproducts is produced; and in addition, exhaust emission in the continuous reaction is greatly reduced, so the environmental pollution is greatly reduced.
Owner:ZHEJIANG QINYAN TECH CO LTD
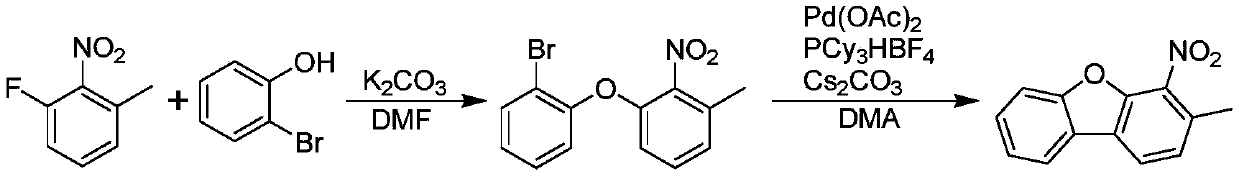
Synthesis method of 3-methyl-4-nitrodibenzofuran
ActiveCN111217779AAvoid costly, lower-yielding problemsClosed ring yield increasedOrganic chemistryPtru catalystOrganic synthesis
The invention discloses a synthesis method of 3-methyl-4-nitrodibenzofuran, and belongs to the technical field of organic synthesis. The synthesis method comprises the following steps: S1, reacting o-chlorophenol with an alkali I to form a salt, then performing a nucleophilic substitution reaction on the salt and 2-nitro-3-chlorotoluene to obtain a mixture I, and performing post-treatment on the mixture I to obtain an intermediate 1; and S2, dissolving the intermediate 1 and 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride in an aprotic polar solvent, performing an intramolecular cyclizationreaction under the action of a Pd catalyst and an alkali II to obtain a mixture II, and performing post-treatment on the mixture II to obtain 3-methyl-4-nitrodibenzofuran. The synthesis method has the advantages of simplicity, cheap raw materials, easiness in purification, and avoiding of the problems of expensive raw materials and low yield in the traditional method.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
The preparation method of clobazam
ActiveCN106749052BAvoid it happening againHigh purityOrganic chemistryLithium hydroxidePotassium hydroxide
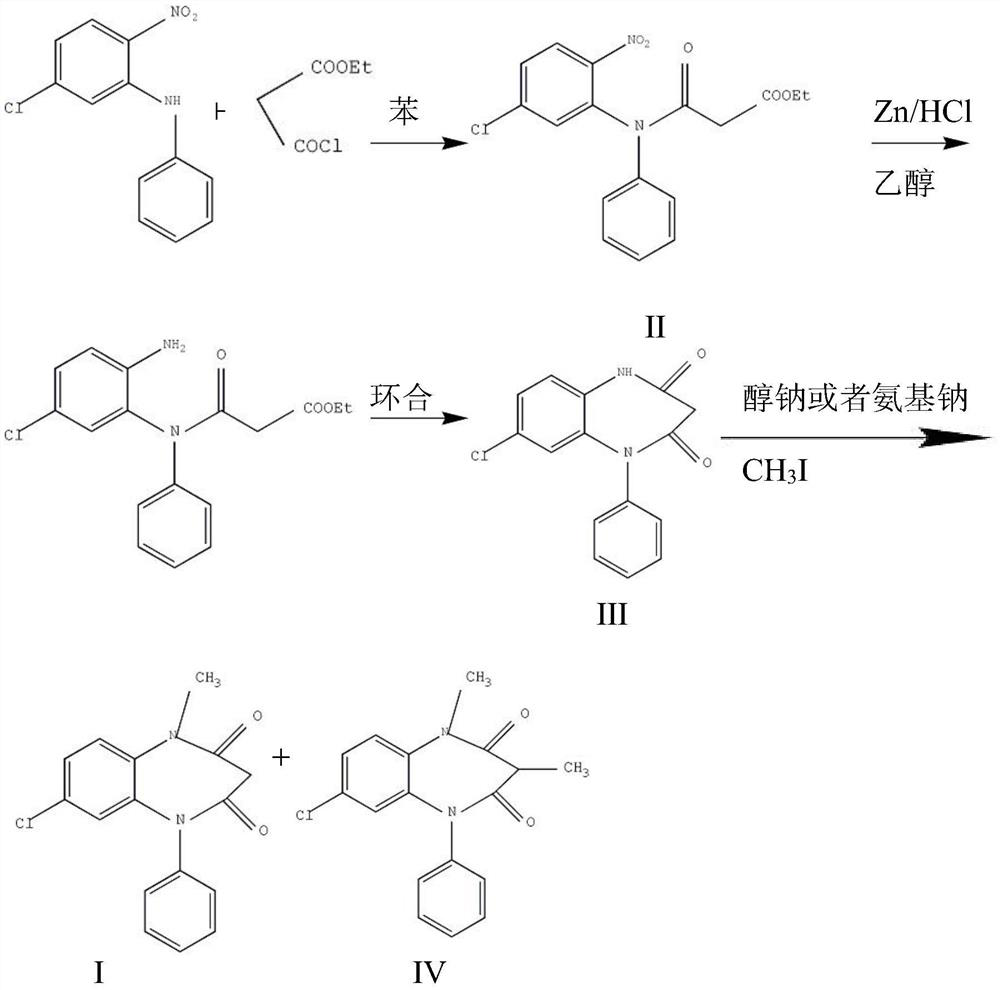
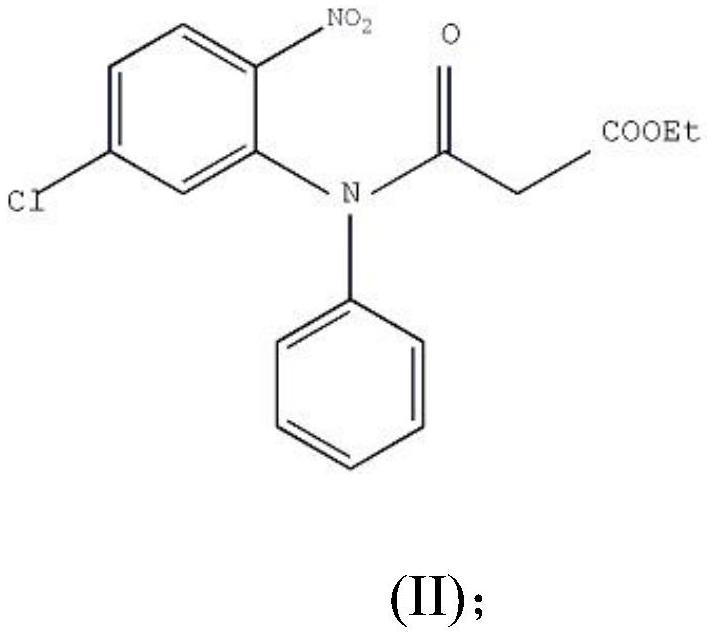
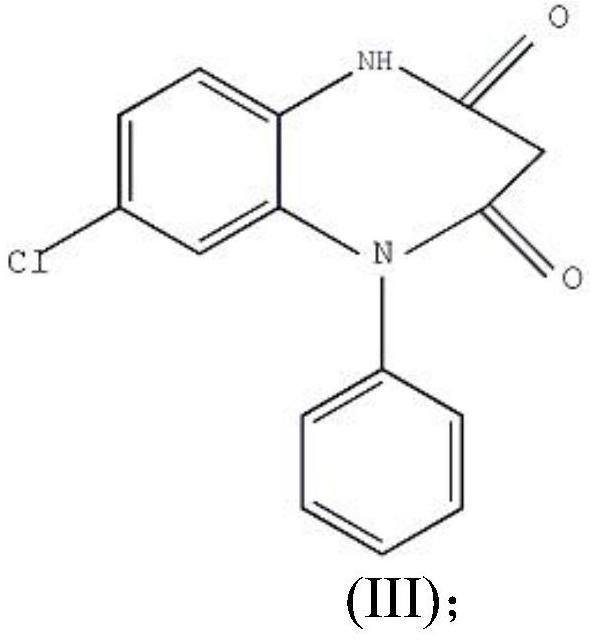
The invention provides a preparation method of clobazam. The preparation method comprises the following steps: (1), using 2-nitro-5-chlorodiphenylamine and ethyl malonyl chloride as raw materials, performing a reflow reaction in an organic solvent, after the reaction, decompressing the organic solvent in the reaction system, then performing evaporation to dryness, and adding a refining solvent for refining treatment so as to obtain a compound as shown in a formula II; (2), performing zinc powder reduction on the compound as shown in the formula II, and performing ammonolysis cyclization so as to obtain a compound as shown in a formula III; (3) enabling the compound as shown in the formula III to react with methyl iodide in an alkaline alcohol solution so as to obtain the clobazam, wherein alkali is one or more of sodium hydroxide, lithium hydroxide and potassium hydroxide. The clobazam prepared by the method is few in impurities and high in purity.
Owner:济南科汇医药科技有限公司
Synthetic method of p-nitroanisole
PendingCN112479891AHigh yieldHas a catalytic effectOrganic chemistryOrganic compound preparationPtru catalystP-nitroanisole
The invention relates to the technical field of organic intermediates, and particularly discloses a p-nitroanisole synthesis method. The method includes the steps: S1 dissolving p-nitroanisole, and dissolving sodium hydroxide and methanol in an organic solvent to form a first mixed solution, wherein the molar ratio of sodium hydroxide to methanol is (1.3-2): 8; S2, performing preliminary reaction,wherein p-nitrochlorobenzene is added into the first mixed solution for 2-3 h, and a second mixed solution is formed, and the molar ratio of the p-nitrochlorobenzene to the addition amount of the methanol in S1 is 1: 8; S3, carrying out secondary reaction: a catalyst is added into the second mixed solution, a quaternary ammonium salt catalyst is selected as the catalyst for 4-8 h, and the molar ratio of the addition amount of the catalyst to the addition amount of the methanol in S1 is (0.003-0.006): 8; S4, carrying out post-treatment, and carrying out post-treatment to obtain p-nitroanisole.By adding the quaternary ammonium salt catalyst, the final yield of p-nitroanisole can be improved.
Owner:ZHEJIANG HONGSHENG CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-chloro-10,11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method 2-chloro-10,11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method](https://images-eureka.patsnap.com/patent_img/756dec1c-9b9c-4a3b-b57f-c452c0cc3130/146924DEST_PATH_IMAGE006.PNG)
![2-chloro-10,11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method 2-chloro-10,11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method](https://images-eureka.patsnap.com/patent_img/756dec1c-9b9c-4a3b-b57f-c452c0cc3130/2013105239029100002DEST_PATH_IMAGE003.PNG)
![2-chloro-10,11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method 2-chloro-10,11-Dihydro-11-oxodibenzo [b, f] [1, 4] thiazepine-11 (10-H)-one preparing method](https://images-eureka.patsnap.com/patent_img/756dec1c-9b9c-4a3b-b57f-c452c0cc3130/2013105239029100002DEST_PATH_IMAGE005.PNG)
![A kind of synthetic method of 1-hydroxyl-pyrrolo[2,3-c]piperidine A kind of synthetic method of 1-hydroxyl-pyrrolo[2,3-c]piperidine](https://images-eureka.patsnap.com/patent_img/28a9a49d-6bc1-4bac-a5c1-3462ea6eabd7/BDA0002353260920000011.png)
![A kind of synthetic method of 1-hydroxyl-pyrrolo[2,3-c]piperidine A kind of synthetic method of 1-hydroxyl-pyrrolo[2,3-c]piperidine](https://images-eureka.patsnap.com/patent_img/28a9a49d-6bc1-4bac-a5c1-3462ea6eabd7/BDA0002353260920000021.png)
![A kind of synthetic method of 1-hydroxyl-pyrrolo[2,3-c]piperidine A kind of synthetic method of 1-hydroxyl-pyrrolo[2,3-c]piperidine](https://images-eureka.patsnap.com/patent_img/28a9a49d-6bc1-4bac-a5c1-3462ea6eabd7/BDA0002353260920000022.png)