O-chloroaniline preparation method
A technology of o-chloroaniline and o-nitrochlorobenzene, applied in the field of preparation of o-chloroaniline, can solve the problems affecting the output and purity of o-chloroaniline, low yield of alkali sulfide reduction method, low vapor-liquid mass transfer performance, etc. , to achieve the effect of inhibiting dechlorination side reaction, stable activity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
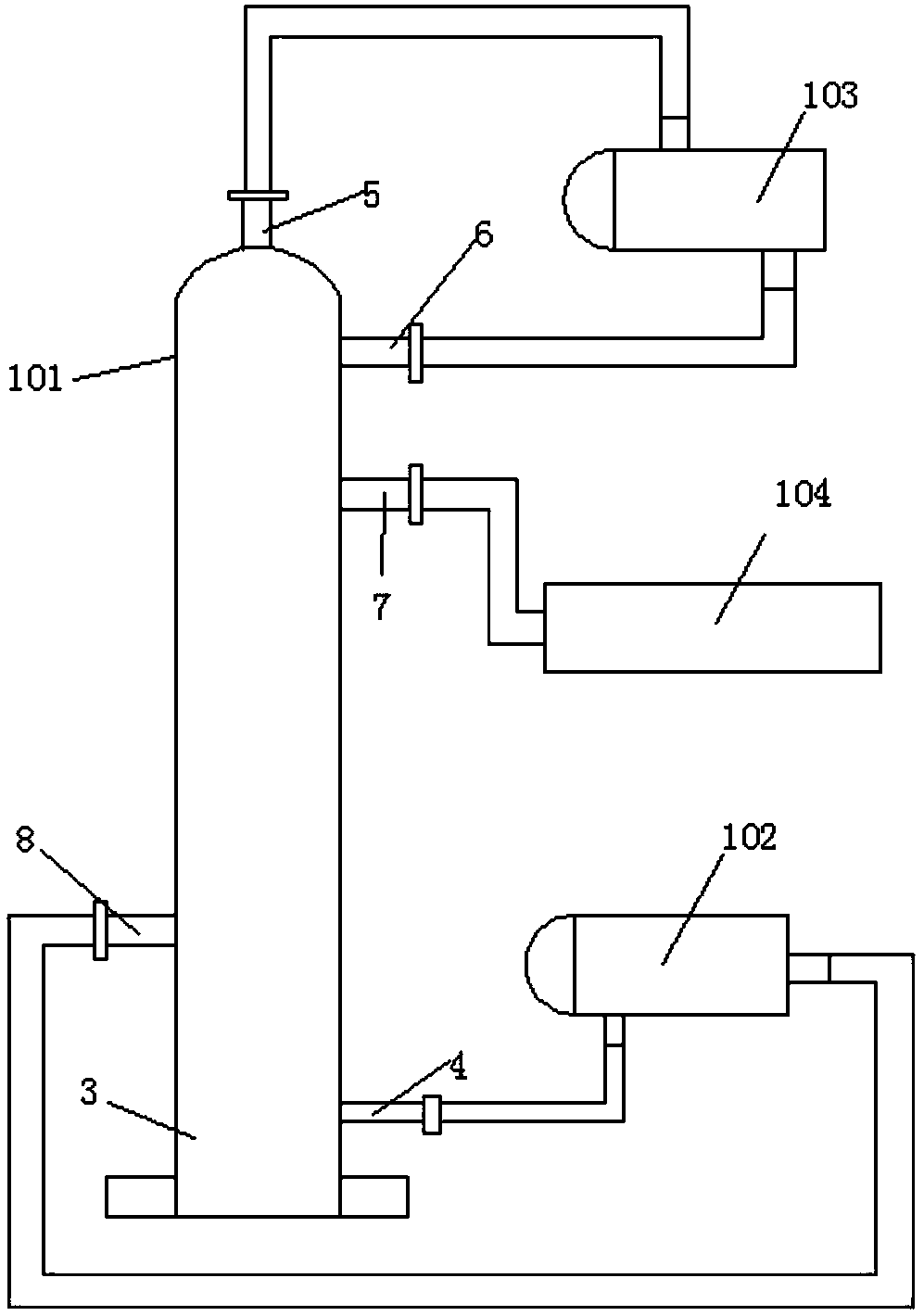
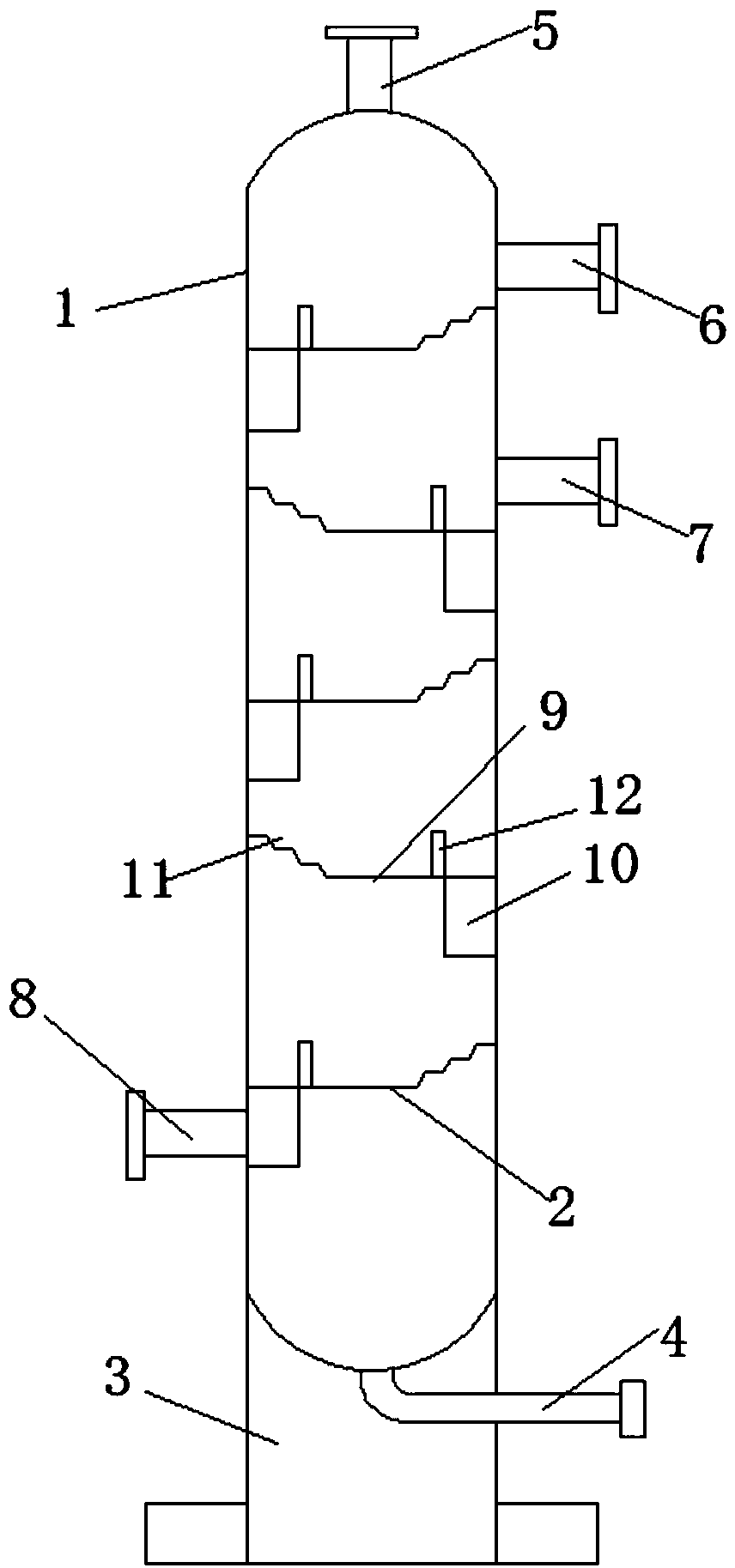
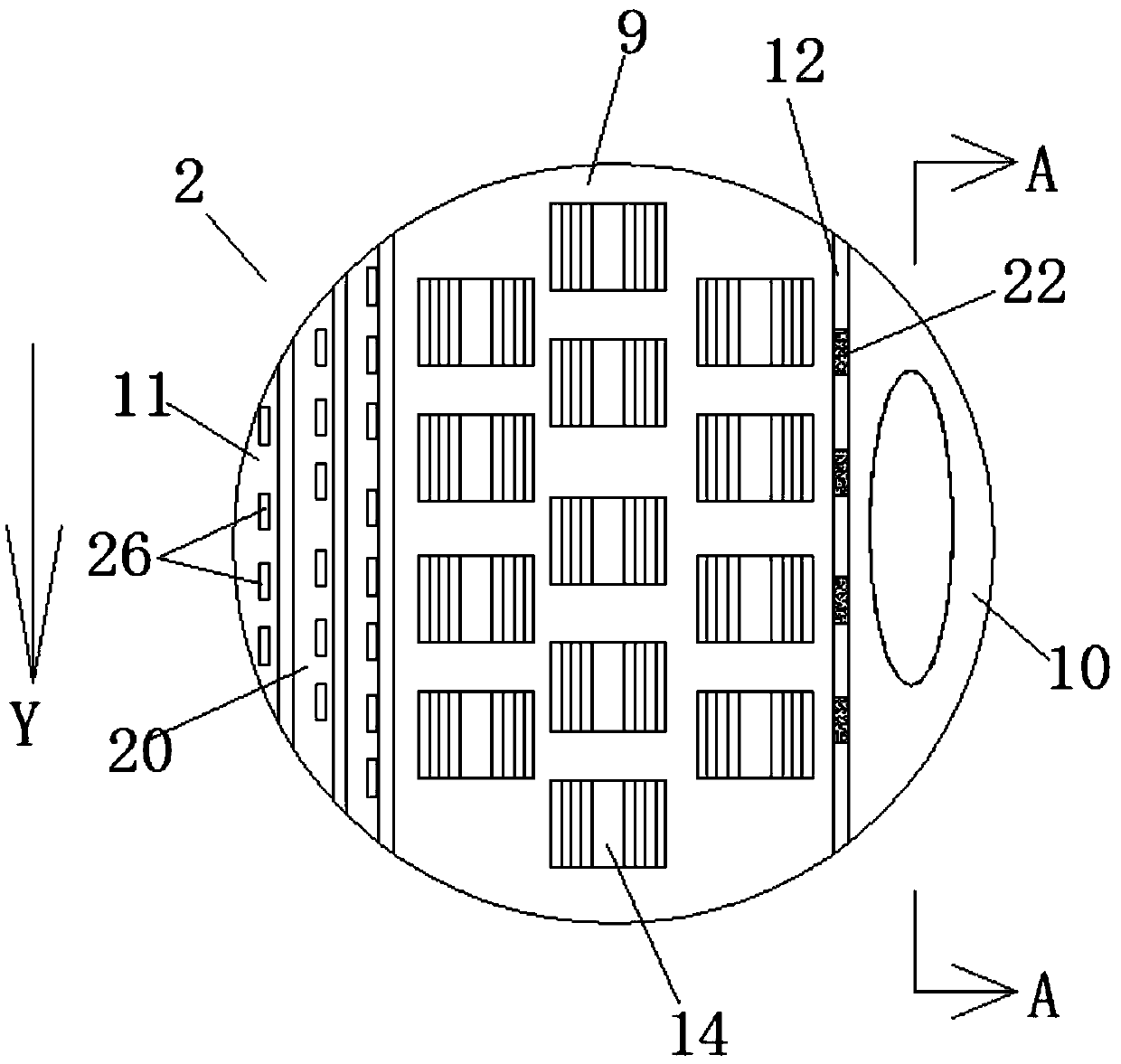
[0036] A preparation method of o-chloroaniline, comprising the following raw materials: o-nitrochlorobenzene, catalyst, dicyandiamide, solvent, nitrogen (steel cylinder), hydrogen (steel cylinder);
[0037] The preparation method is as follows:
[0038] Step (1): Weigh 80-200g of o-nitrochlorobenzene, 20-50g of catalyst, 3-6g of dicyandiamide, and 800-2000ml of solvent based on parts by weight;
[0039] Step (2) Initial mixing: adding o-nitrochlorobenzene and solvent into the autoclave, and then adding catalyst and dicyandiamide in sequence;
[0040] Step (3): Seal the autoclave, first replace the air in the autoclave with nitrogen 3-7 times, then replace the nitrogen in the autoclave with hydrogen, and increase the pressure to 0.8MPa-2MPa; maintain the pressure unchanged after increasing the pressure, stir and heat Warm up to 60-120℃, timed reaction for 2-5 hours
[0041] In step (4): after the reaction, add cooling water to cool down and release the pressure. After the pressure relea...
Embodiment 2
[0053] It is basically the same as the above embodiment, and the difference lies in:
[0054] The preparation method is as follows:
[0055] Step (1): Weigh 200 g of o-nitrochlorobenzene, 50 g of catalyst, 6 g of dicyandiamide, and 2000 ml of methanol in parts by weight;
[0056] Step (2) Initial mixing: adding o-nitrochlorobenzene and solvent into the autoclave, and then adding catalyst and dicyandiamide in sequence;
[0057] Step (3): Seal the autoclave, first replace the air in the autoclave with nitrogen 7 times, then replace the nitrogen in the autoclave with hydrogen, and increase the pressure to 0.8MPa; after the pressure is increased, maintain the pressure unchanged, stir and heat up to 100°C , Timing response 4 hours
[0058] In step (4): after the reaction, add cooling water to cool down and release the pressure. After the pressure release is completed, take out the upper layer material in the autoclave and pour it into a rectification device for rectification. After rectific...
Embodiment 3
[0062] It is basically the same as the above embodiment, and the difference lies in:
[0063] The preparation method is as follows:
[0064] Step (1): Weigh 100 g of o-nitrochlorobenzene, 20 g of catalyst, 5 g of dicyandiamide, and 1000 ml of methanol in parts by weight;
[0065] Step (2) Initial mixing: adding o-nitrochlorobenzene and methanol into the autoclave, and then adding catalyst and dicyandiamide in sequence;
[0066] Step (3): Seal the autoclave, first replace the air in the autoclave with nitrogen 6 times, then replace the nitrogen in the autoclave with hydrogen, and increase the pressure to 1.2MPa; after the increase, keep the pressure constant, stir and heat to raise the temperature to 100°C , Timed response for 2 hours;
[0067] In step (4): after the reaction, add cooling water to cool down and release the pressure. After the pressure release is completed, take out the upper layer material in the autoclave and pour it into a rectification device for rectification. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com