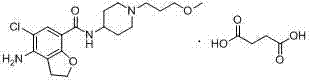
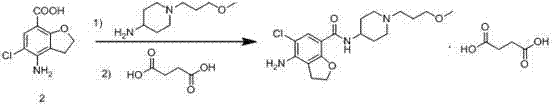
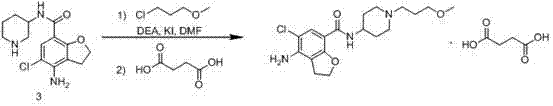
Method for preparing prucalopride
An organic solvent, dihydrobenzene technology, applied in the field of medicinal chemistry, can solve the problems of unsuitability for industrial production, low yield, long reaction route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve 12.1 g of 4-nitro-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid in 50 mL of tetrahydrofuran, add 8.1 g of CDI, and add 1-(3-methoxypropyl )-4-Piperidine ammonia 8.6g, heated up to 80°C to react, TLC detected the reaction, cooled to room temperature after the reaction, added 200mL of water, solid washed out, filtered to obtain N-[1-(3-methoxypropane Base)-4-nitro-5-chloro-2,3-dihydrobenzofuran-7-carboxamide, the yield was 95%.
Embodiment 2
[0021] Dissolve 12.1 g of 4-nitro-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid in 50 mL of N,N-dimethylformamide, add 8.1 g of CDI, and add 1- (3-Methoxypropyl)-4-piperidinamine 8.6g, heated up to 80°C to react, TLC detected the reaction, cooled to room temperature after the reaction, added 200mL of water, solid washed out, filtered to obtain N-[ 1-(3-methoxypropyl)-4-nitro-5-chloro-2,3-dihydrobenzofuran-7-carboxamide, the yield was 85%.
Embodiment 3
[0023] Dissolve 12.1 g of 4-nitro-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid in 50 mL of dichloromethane, add 8.1 g of CDI, and add 1-(3-methoxy Propyl)-4-piperidinium 8.6g, react at room temperature, TLC detects the reaction, after the reaction is completed, cool to room temperature, add 200mL of water, there is solid washing out, filter to get N-[1-(3-methoxypropyl )-4-nitro-5-chloro-2,3-dihydrobenzofuran-7-carboxamide, the yield is 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com