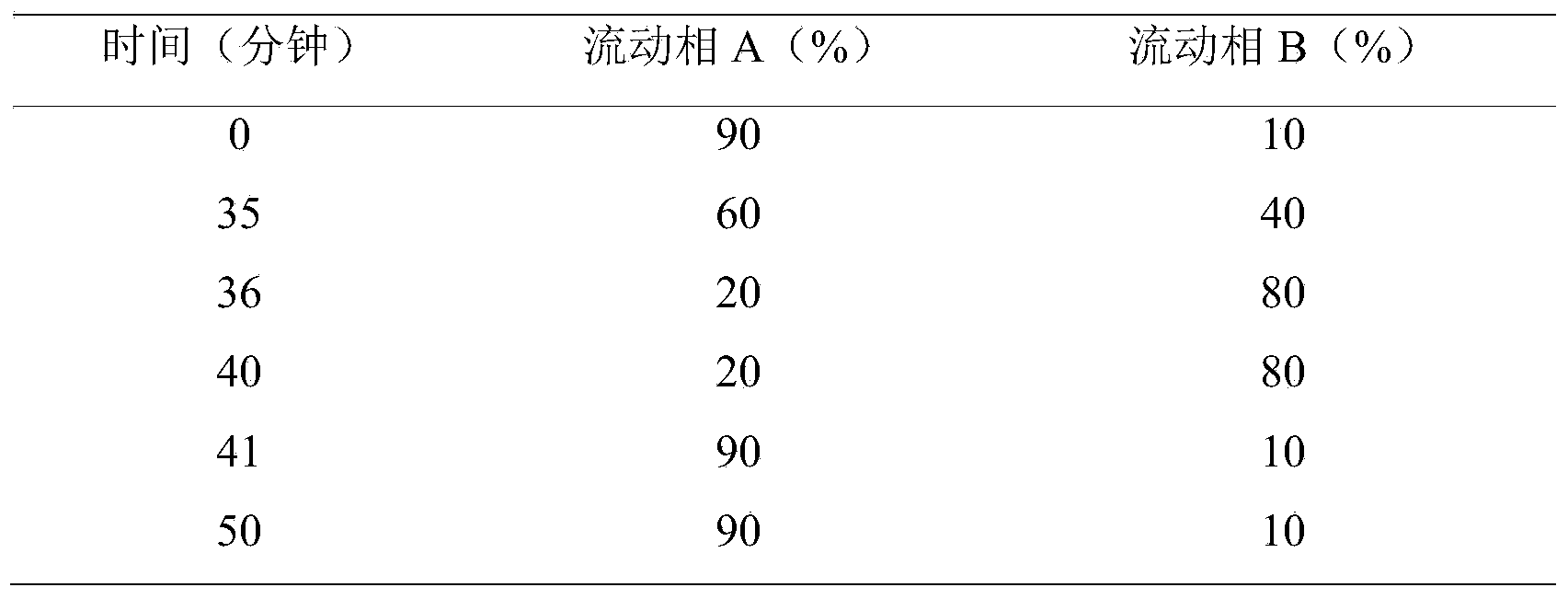
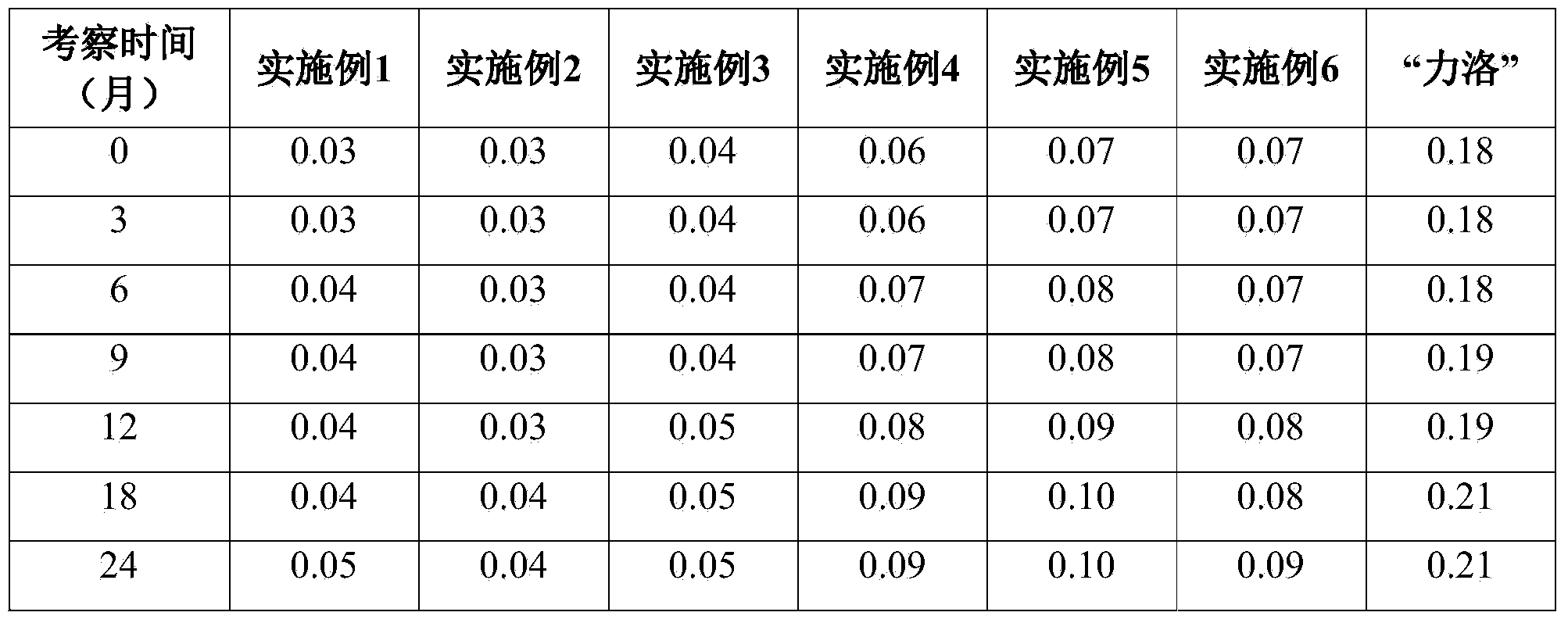
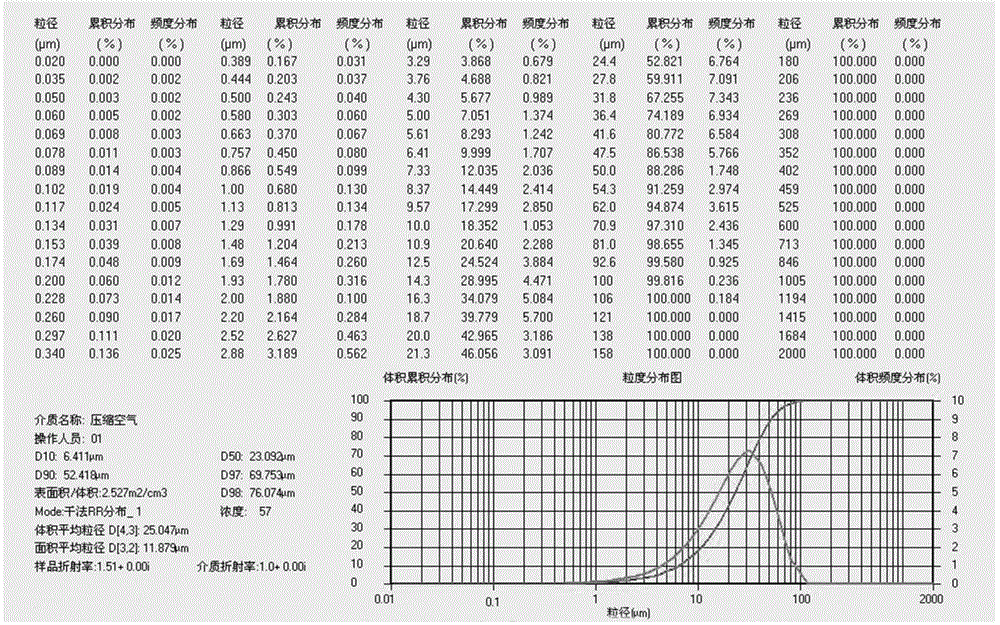
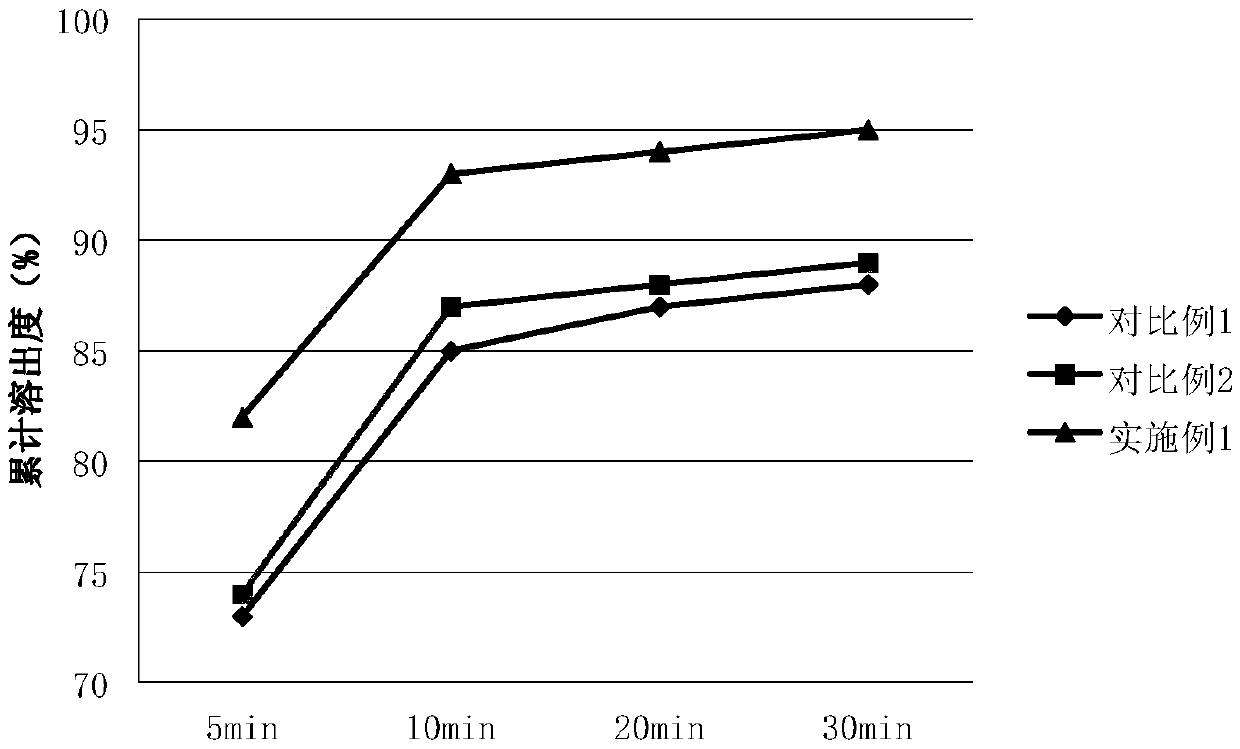
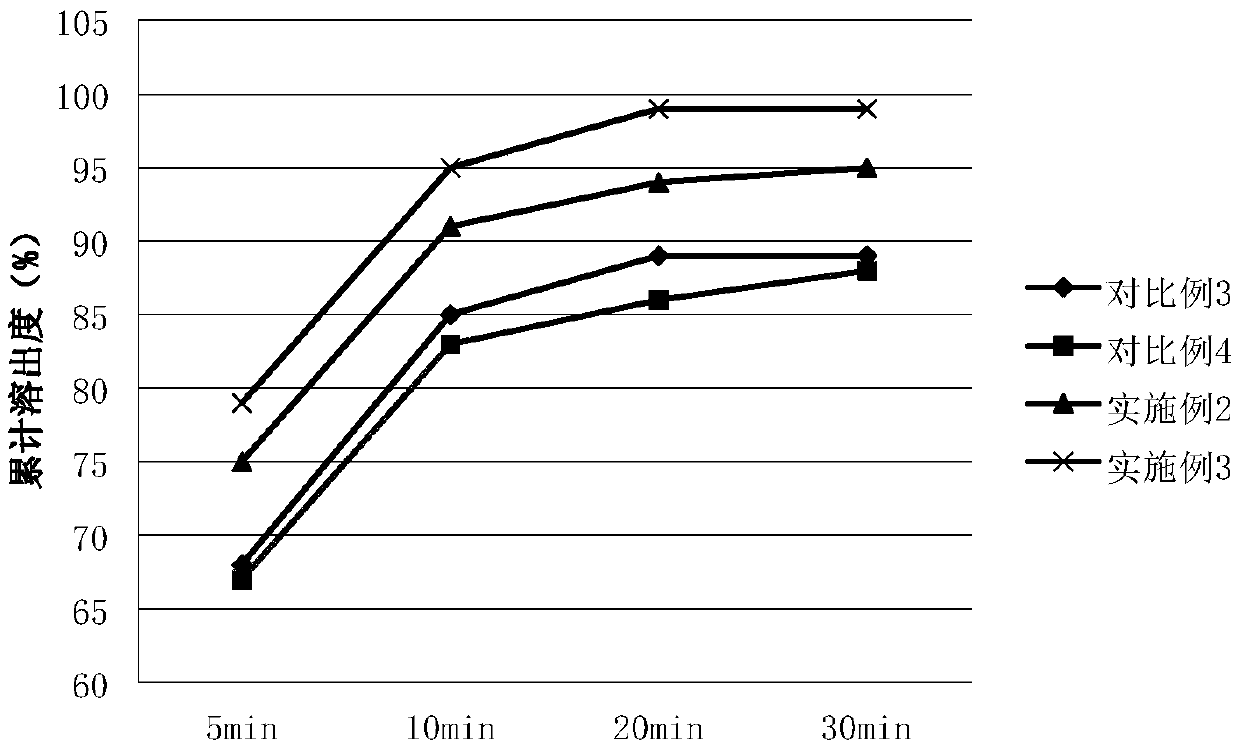
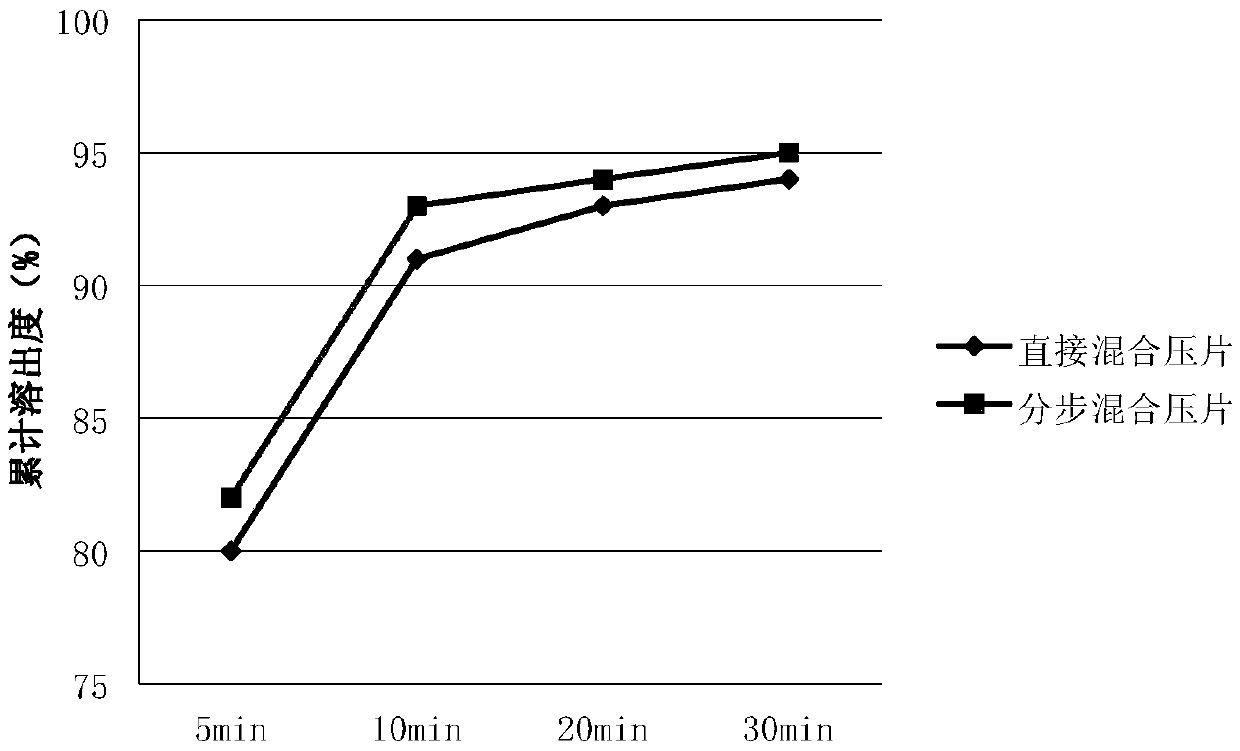
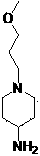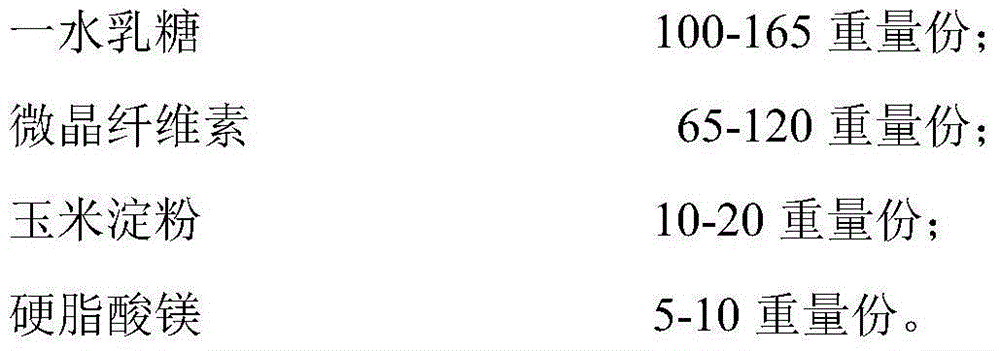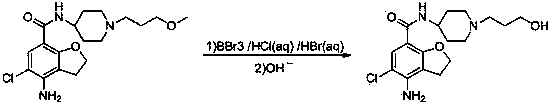Patents
Literature
43 results about "Prucalopride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
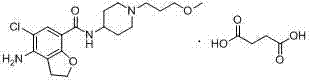
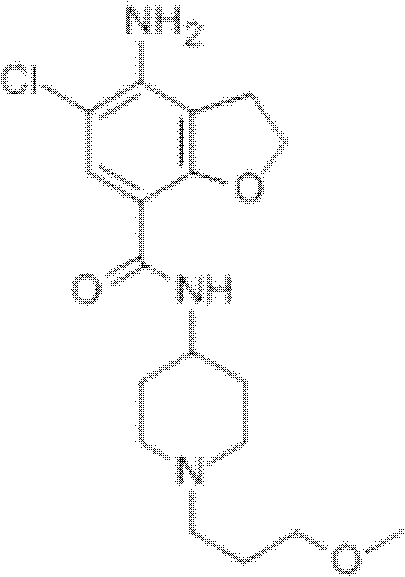
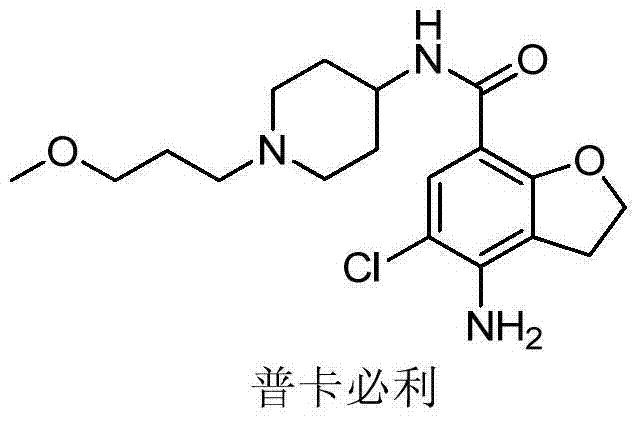
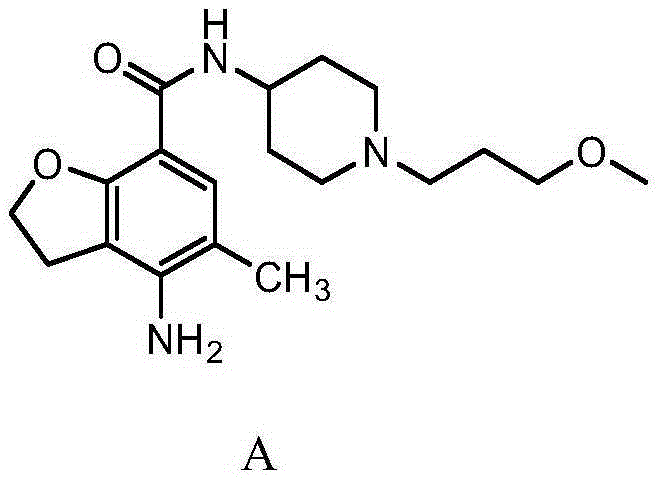
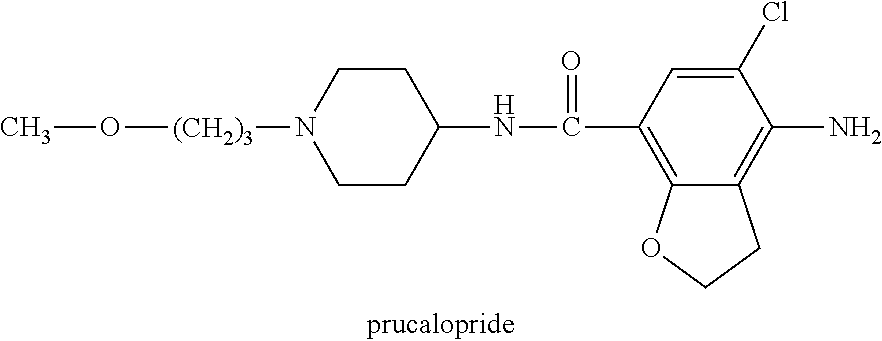
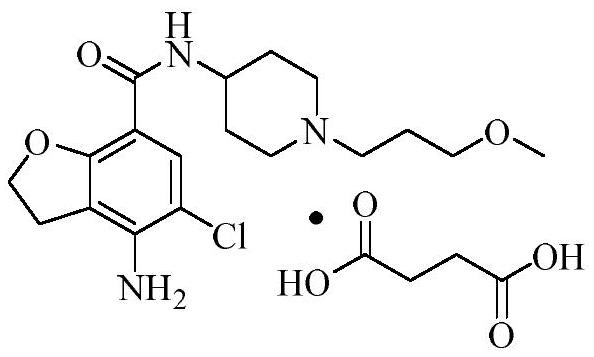
Prucalopride, brand name Prudac, among others, is a drug acting as a selective, high affinity 5-HT₄ receptor agonist which targets the impaired motility associated with chronic constipation, thus normalizing bowel movements. Prucalopride was approved for use in Europe in 2009, in Canada in 2011 and in Israel in 2014 but has only been recently approved by the Food and Drug Administration for use in the United States. The drug has also been tested for the treatment of chronic intestinal pseudo-obstruction.
Therapy for enteric infections
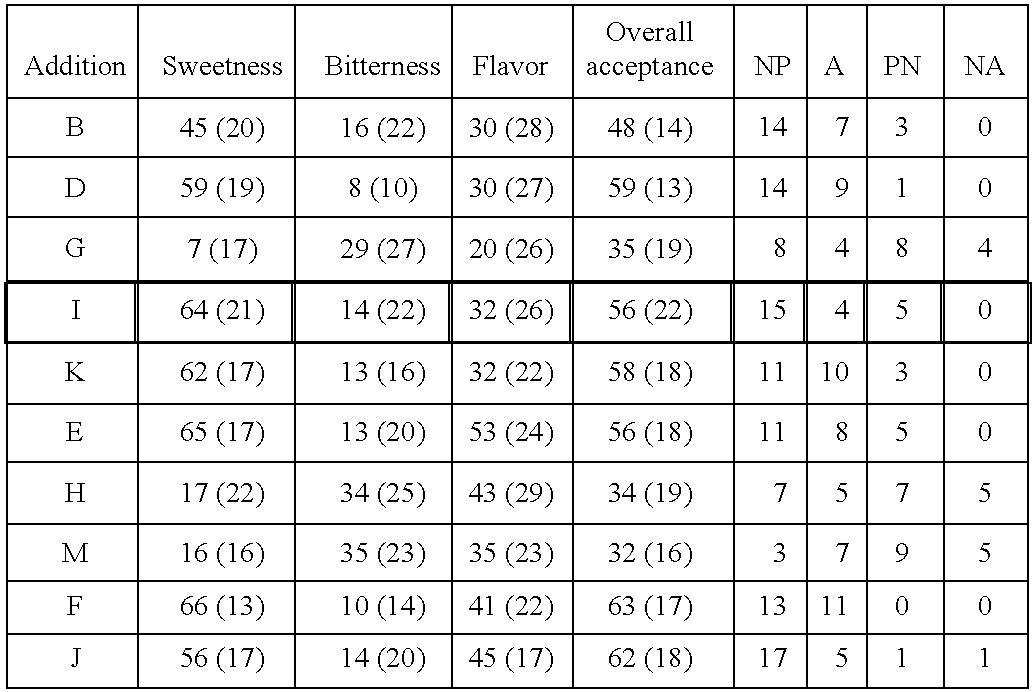
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhea, chronic idiopathic nausea, IBD-associated constipation and diarrhea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
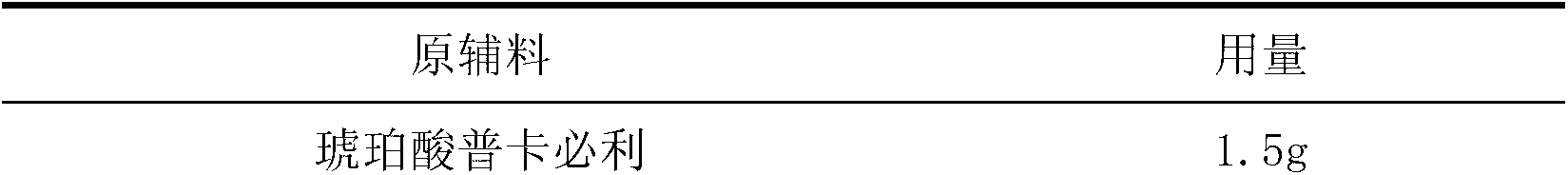
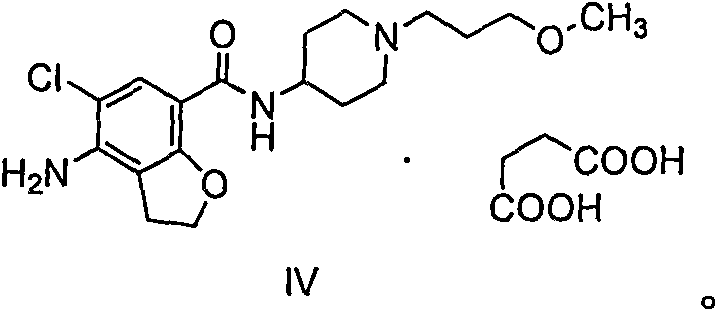
Oral solid preparation taking prucalopride succinate as active ingredient and application of oral solid preparation
The invention provides oral solid preparation taking prucalopride succinate as an active ingredient, and application of the oral solid preparation. According to the method, the oral solid preparation is prepared by taking prucalopride succinate and pharmaceutically acceptable auxiliary materials and adopting the preparation technology. The solid preparation is stable in quality, controllable, convenient to take, good in compliance, and less in side effects, and is mainly used for chronic constipation in clinic practice.
Owner:FUKANGREN BIO PHARMA
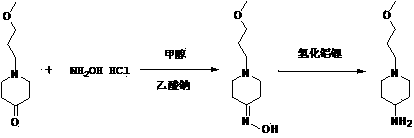
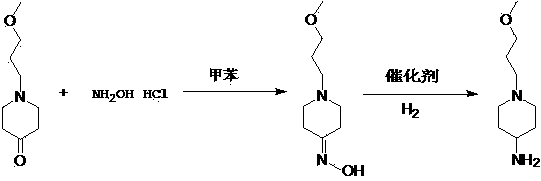
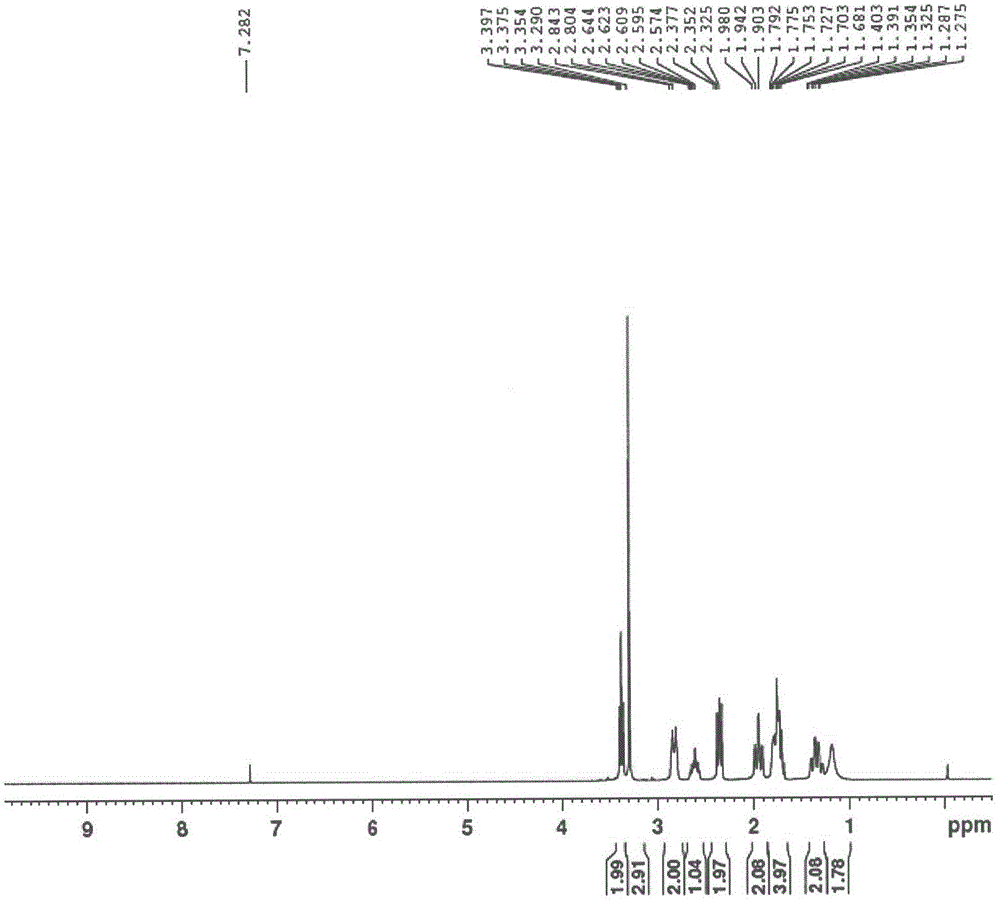
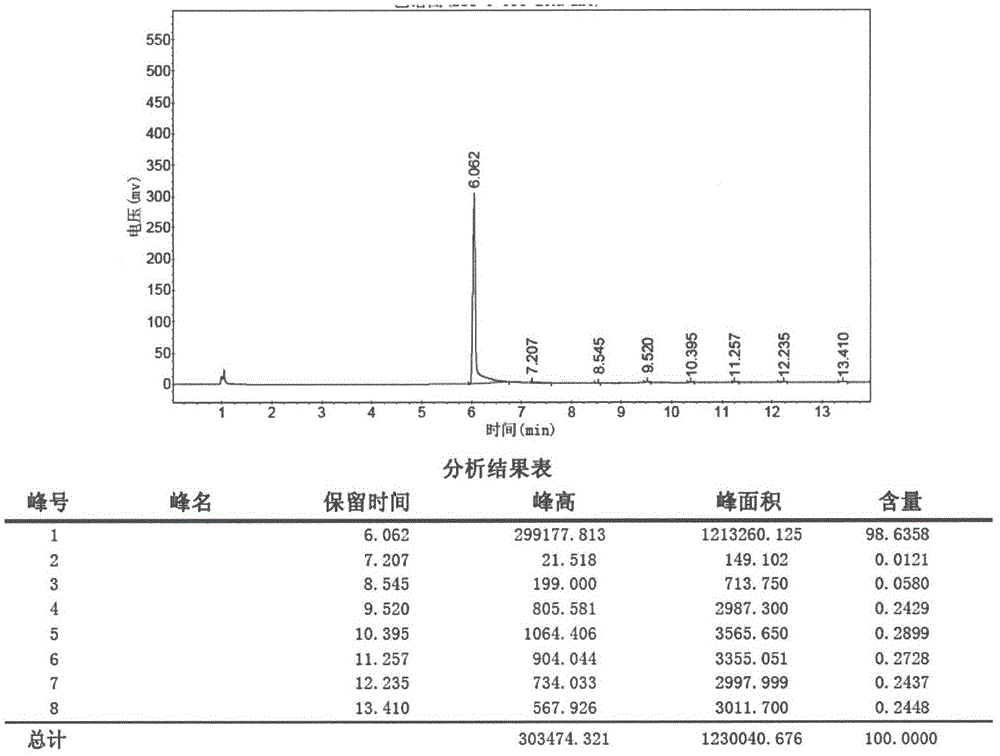
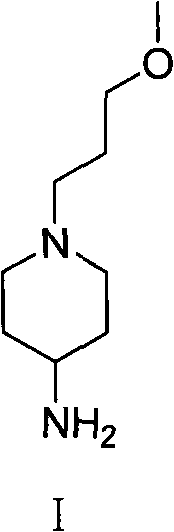
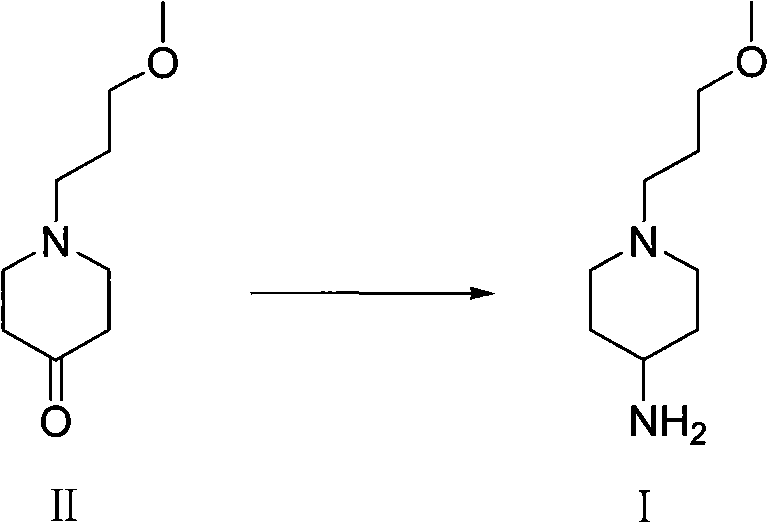
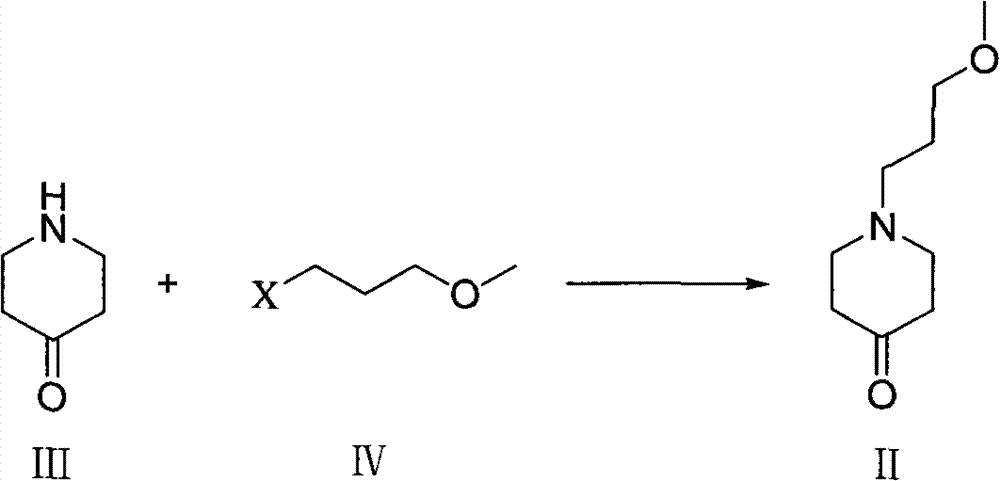
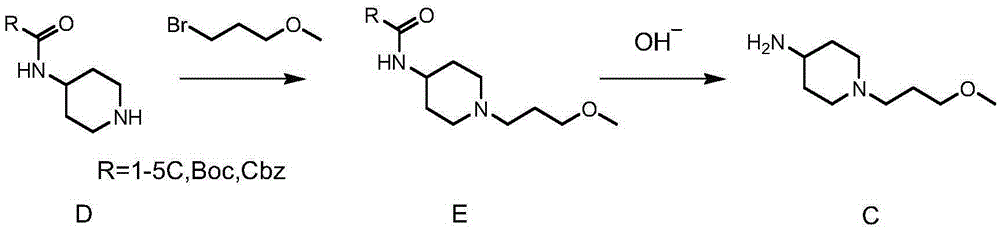
Method used for preparing prucalopride intermediate 1-(3-methoxypropyl)-4-piperidinamine
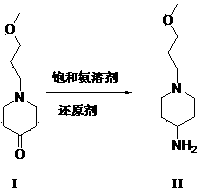
The invention belongs to the field of pharmaceutical chemistry, and relates to a method used for preparing prucalopride intermediate 1-(3-methoxypropyl)-4-piperidinamine. The method comprises following step: 1) 1-(3-methoxylpropyl)-4-piperidone is dissolved in an organic solvent, appropriate amounts of hydroxylamine hydrochloride and vinegar are added for heating reflux dehydration so as to obtain 1-(3-methoxypropyl)-4-piperidine oxime; and 2) 1-(3-methoxypropyl)-4-piperidine oxime is dissolved in an organic solvent, an appropriate amount of a catalyst is added, and H2 is added so as to obtain 1-(3-methoxypropyl)-4-piperidinamine after complete reaction. Operation of the method is simple and convenient, reaction conditions are mild, yield is high, and the method is suitable for large scale industrialized production.
Owner:BEIJING VENTUREPHARM BIOTECH
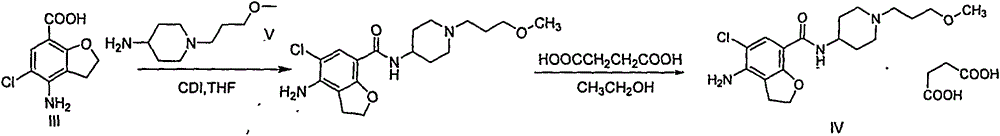
Method for preparing prucalopride
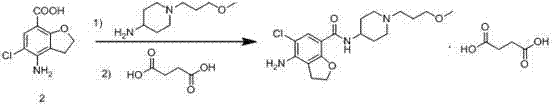
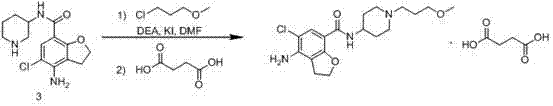
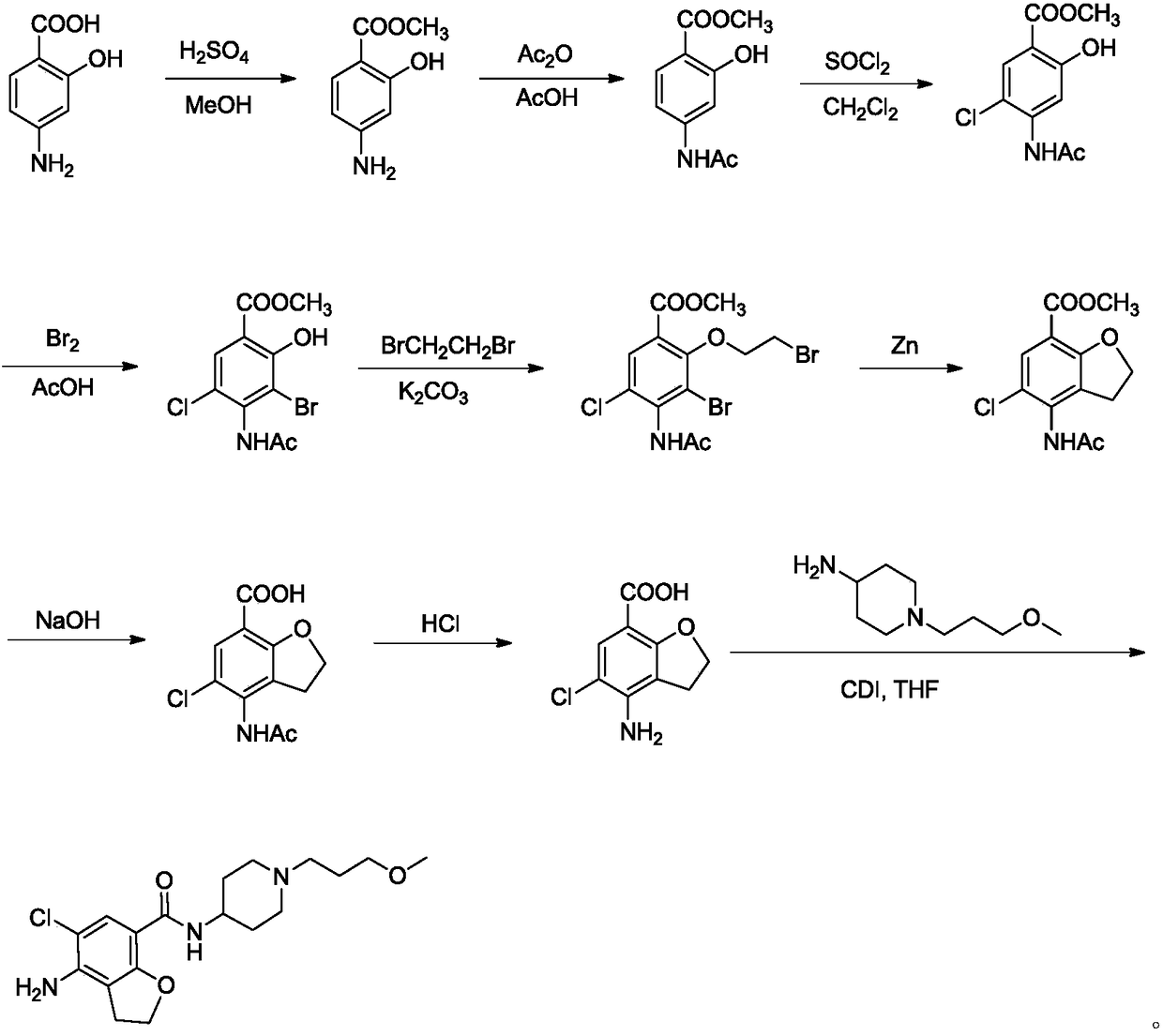
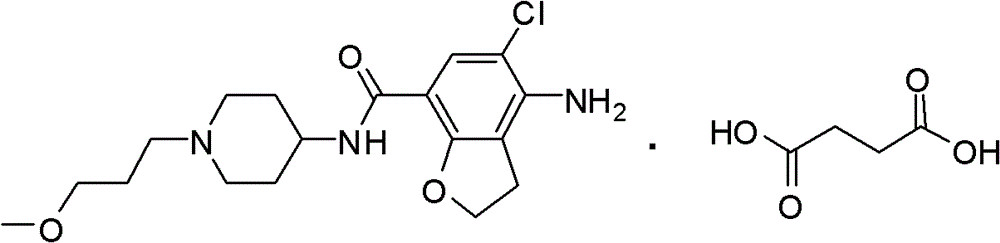
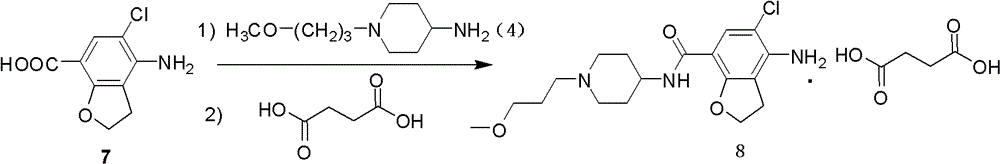
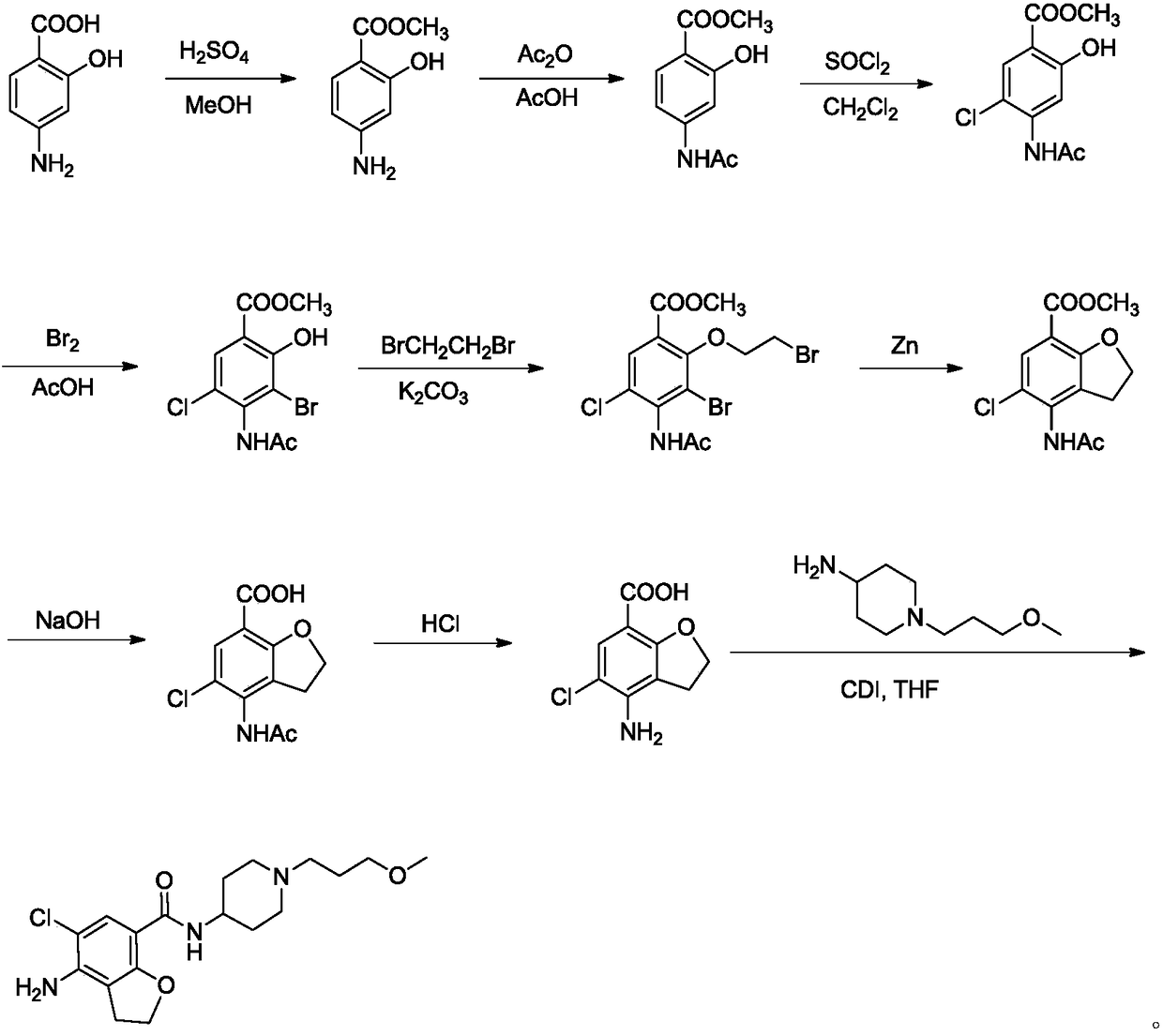
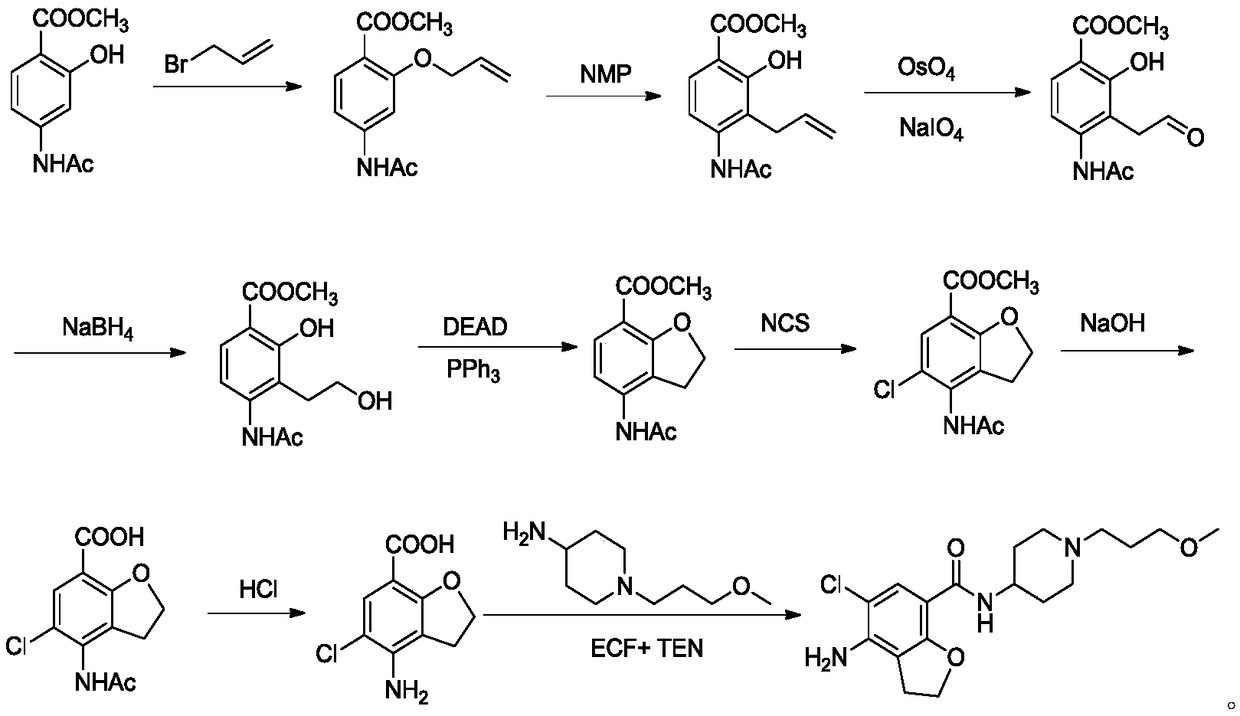
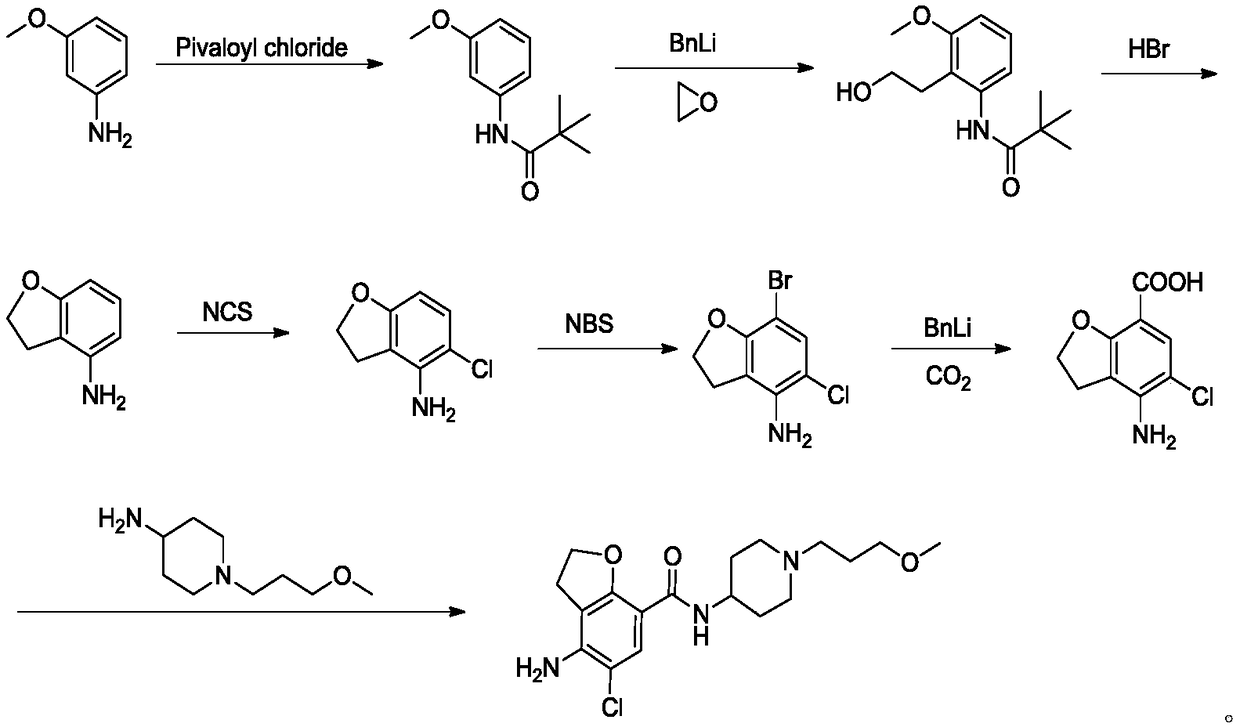
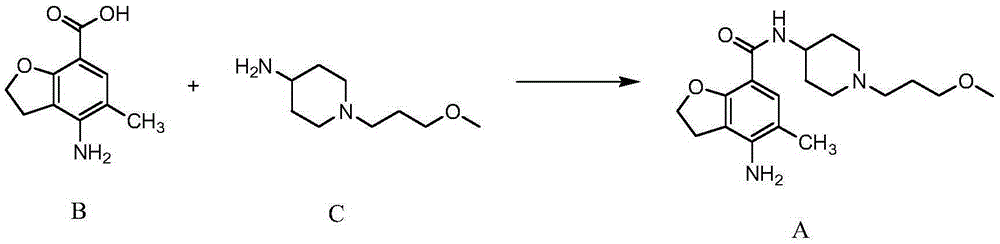
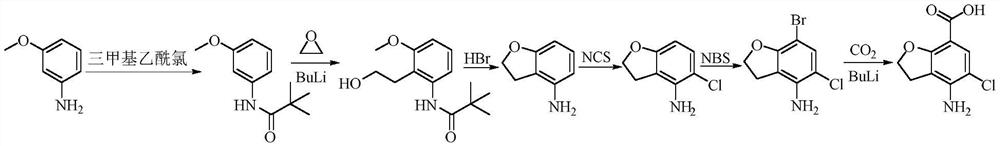
The invention belongs to the field of medicinal chemistry, and in particular relates to a preparation method of prucalopride. Prucalopride succinate is a 5-HT4 receptor stimulant with high selectivity and specificity, and is a novel intestinal motility drug. The drug has the characteristics of high selectivity, rapid onset and less untoward effect, and has wide clinical application prospect in the field of constipation treatment. The invention provides a new method for compounding prucalopride, wherein 4-nitro-5-chlor-2,3-dihydrobenzofuran-7-methanoic acid and 1-(3-methoxypropyl)-4-piperidinamine are employed as initial materials to prepare prucalopride; and the preparation method is simple and convenient to operate, less in side reaction, high in yield, mild in reaction condition and convenient for large-scale industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation method of Prucalopride intermediates
The invention belongs to the field of medicine chemistry, and specifically relates to a preparation method of Prucalopride intermediates 1-(3-Methoxypropyl)-4-piperidinamine. The preparation method comprises following steps: dissolving 1-(3-Methoxypropyl)-4-piperidone (I) in an organic solvent, adding in saturated ammonia solution or ammonia salt and a reducing agent so as to obtain 1-(3-Methoxypropyl)-4-piperidinamine (II). The preparation method of Prucalopride intermediates has the advantages of simple operation, mild reaction conditions, high yield, and suitability for mass industry production.
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation method of prucalopride succinate
ActiveCN108976216AReduce usageGreen environmental protection and low toxicityOrganic chemistryAromatic hydrocarbonNucleophilic substitution
The invention provides a preparation method of prucalopride succinate. The method comprises the steps that 4-nitrobenzaldehyde is taken as an initial raw material, and protected by formyl group, nitroreduction, amino protection, aromatic hydrocarbon chlorination, nucleophilic substitution, molecular internal cyclization, chlorination, deprotection and oxidative amidation are conducted sequentially, and prucalopride succinate is synthesized. The preparation method is safe in technology, no highly toxic reagents is used, the preparation method is green and environmentally friendly, the by-products generated in the reaction are less, and the yield is improved.
Owner:IANGSU COLLEGE OF ENG & TECH
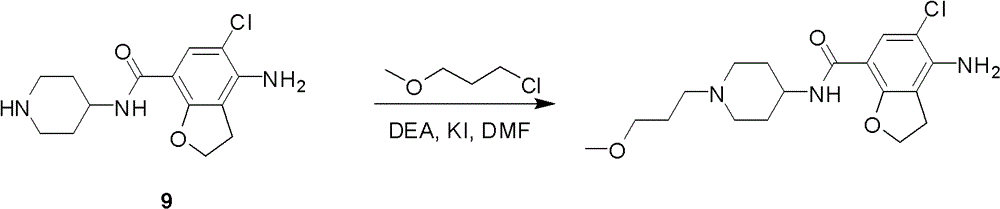
4-n-substituted-1-(3-methoxypropyl)-4-piperidinamine compounds and their preparation and application
InactiveCN102295594BEasy to manufactureMeeting the needs of the pharmaceutical industryOrganic chemistryPharmaceutical industrySuccinic acid
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Novel process for preparing prucalopride intermediate
InactiveCN106146386AFull ammonolysisSuitable for industrial productionOrganic chemistryProcess engineeringCombinatorial chemistry
The invention discloses a preparation method of 1-(3-methoxypropyl)-4-piperidinamine shown as formula (I). The preparation method includes (1), allowing a compound of the formula (II) to react in an ammoniac solution to obtain a compound of the formula (III); (2), allowing the compound of the formula (III) to react with 1,3-dibromo-5,5-dimethylhydantoin under an alkaline condition to obtain the compound of the formula (I). The preparation method has the advantages that special reaction equipment is not needed, and the method is simple and convenient to operate and suitable for industrial production; yield is high, three wastes are few, and cost is low; chemical purity of products is high, and heavy metal residues are avoided.
Owner:JIANGSU VCARE PHARMATECH
Method for preparing 1-( 3-methoxy propyl )- 4-piperidine amine and salt thereof
InactiveCN102898356ARaw materials are cheap and easy to getEasy to operateOrganic chemistryHydrogenAmmonia
The invention discloses a method for preparing 1-(3-methoxy propyl)-4-piperidine amine shown as a formula I. The method comprises the following steps: carrying out the following reactions on a compound shown as a formula II in an organic solution of ammonia, under the effects of hydrogen and a hydrogenation catalyst. The invention also discloses a method for preparing a salt of the 1-( 3-methoxy propyl )-4-piperidine amine shown as the formula I. The method has advantages of cheap and easily available raw materials and convenient operation, is suitable for industrial mass production, and provides a novel path for Prucalopride synthesis.
Owner:SHANGHAI INST OF PHARMA IND +1
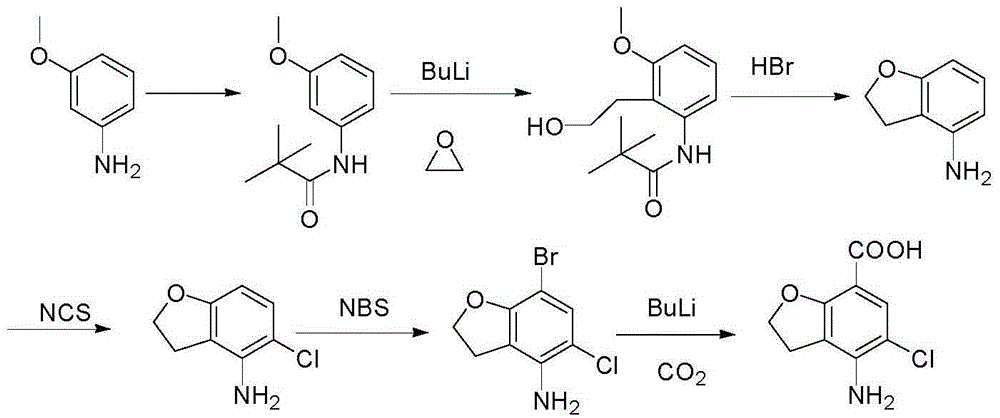
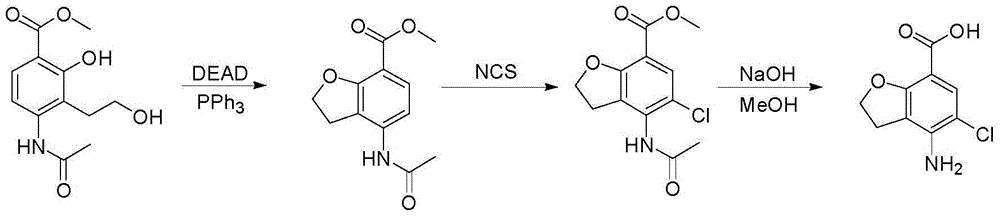
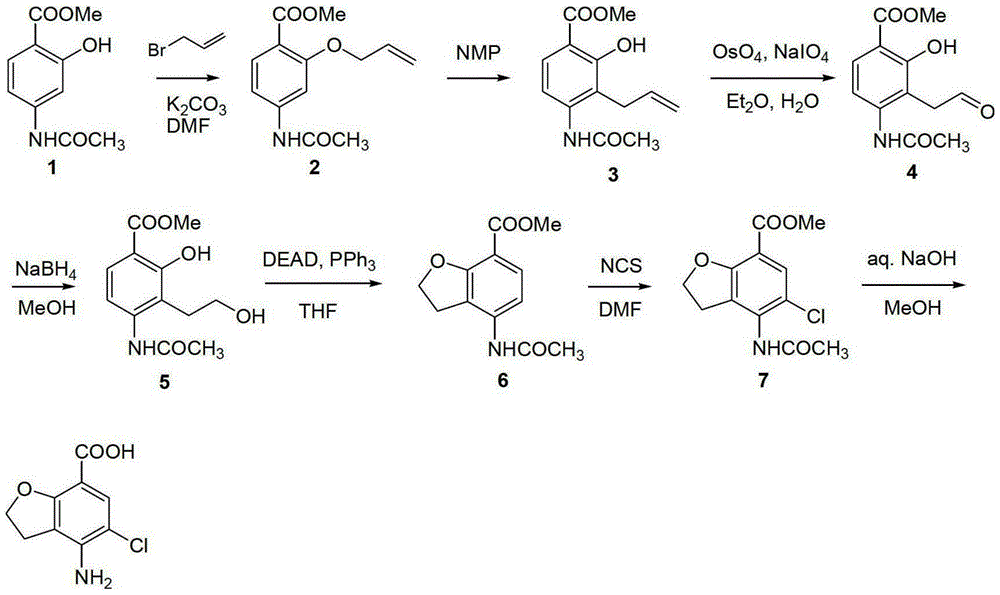
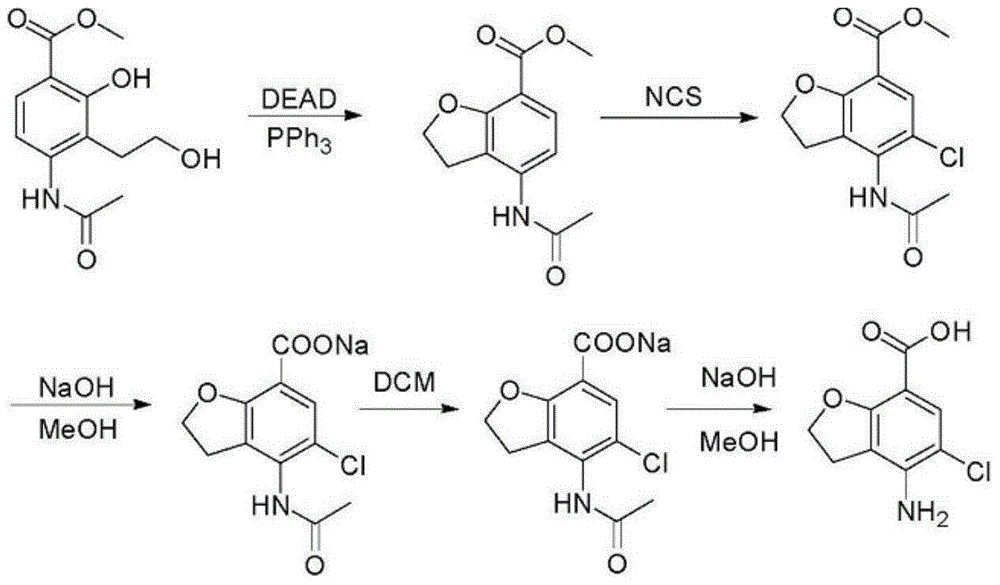
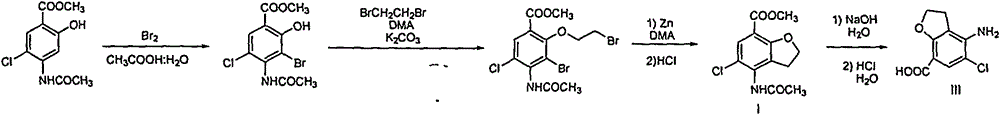
Method for synthesizing 4-amino-5-chloro-2,3-dihydro benzofuran-7-carboxylic acid
InactiveCN104016949AEasy to operate in industrialized productionHigh yieldOrganic chemistryCarboxylic acidSolvent
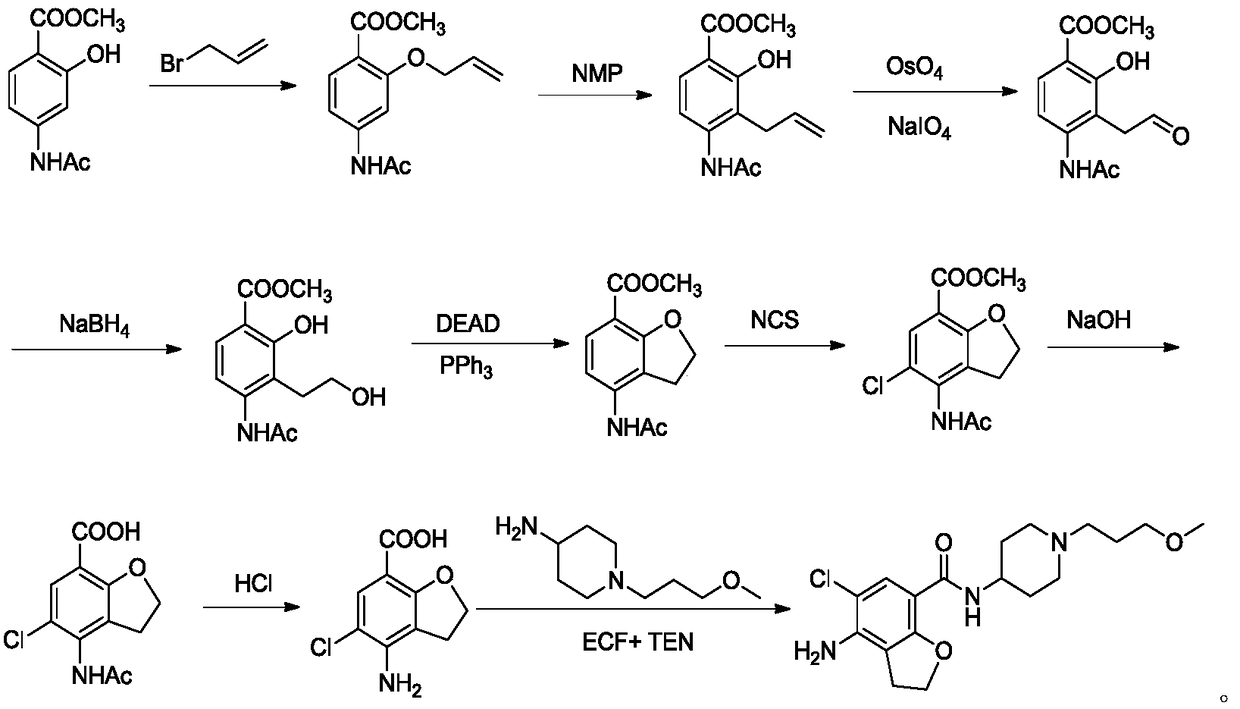
The invention discloses a method for synthesizing a prucalopride midbody 4-amino-5-chloro-2,3-dihydro benzofuran-7-carboxylic acid. The method comprises the following steps: firstly, adding methyl 4-(acetyl amino)-2-hydroxy-3-(2-hydroxy ethyl) benzoate into an organic solvent, adding triphenylphosphine and azo dioctyl phthalate diethyl ester, performing cyclization to obtain a methyl 4-acetamido-2,3-dihydro benzofuran-7-formate rough product, directly chloridizing the rough product by using N-chloro succinimide to obtain a rough product methyl 4-acetamide amino-5-chloro-7-benzofuran formate, and performing hydrolysis and purification to obtain a 4-amino-5-chloro-2,3-dihydro benzofuran-7-carboxylic acid pure product. Compared with a conventional method, the method simplifies the industrial production difficulty and remarkably improves the yield, so that the method disclosed by the invention is easy in industrial production operation, nearly no organic and inorganic waste solvents are used, the total yield is relatively high, and the industrial on-scale production is facilitated.
Owner:TIANJIN WEIJIE TECH
Method for preparing prucalopride intermediate 1-(3-methoxypropyl)-4-piperidylamine
The invention belongs to the medicinal chemistry field and relates to a method for preparing a prucalopride intermediate 1-(3-methoxypropyl)-4-piperidylamine. The prucalopride is clinically used for treating female constipation of un-relievable hypocatharsis, and is the first dihydrobenzofuran carboxylic acid type novel intestinal motility accelerant. In lots of prucalopride synthetic routes, the 1-(3-methoxypropyl)-4-piperidylamine is one of essential intermediates. The invention provides a new route for synthesizing the 1-(3-methoxypropyl)-4-piperidylamine; 4-piperidone is taken as the raw material for the route and the route has the characteristics of easy-to-get raw materials, mild reaction conditions and high product yield; and besides, the method is applicable to industrial production.
Owner:万全万特制药江苏有限公司
Method for preparing prucalopride
ActiveCN109232544AReduce usageGreen environmental protection and low toxicityOrganic chemistryAlkyl transferMethylaniline
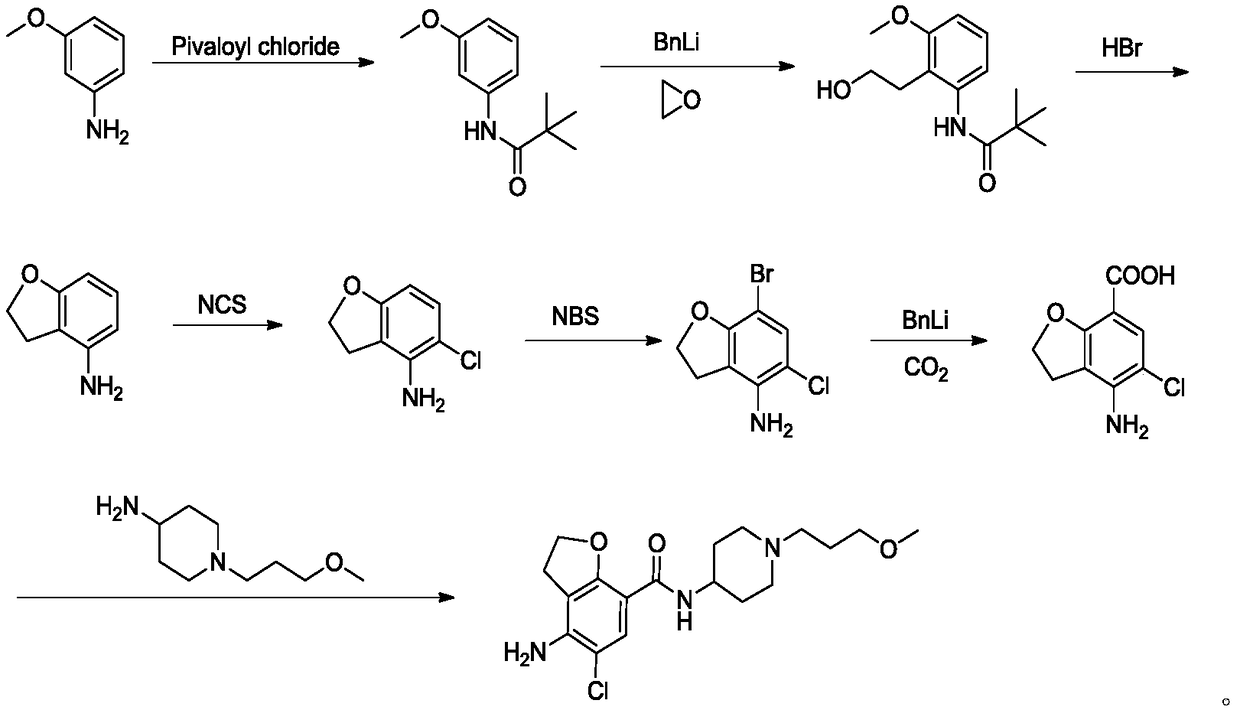
The invention provides a method for preparing prucalopride. The method comprises the following steps: taking 3-chloro-4-methylaniline as an initial raw material, performing amino protection, nucleophilic substitution, hydroxy protection, aromatic hydrocarbon chlorination, free radical bromination, hydrolysis, molecular Friedel-Crafts alkylation reaction ring closure, amino deprotection and oxidative amidation, thereby obtaining the prucalopride. According to the scheme, a dangerous process is not adopted, any highly toxic reagent is not used, the method is safe, green and environmental-friendly, the amount of by-products produced in the reaction is small, and the yield is improved.
Owner:IANGSU COLLEGE OF ENG & TECH
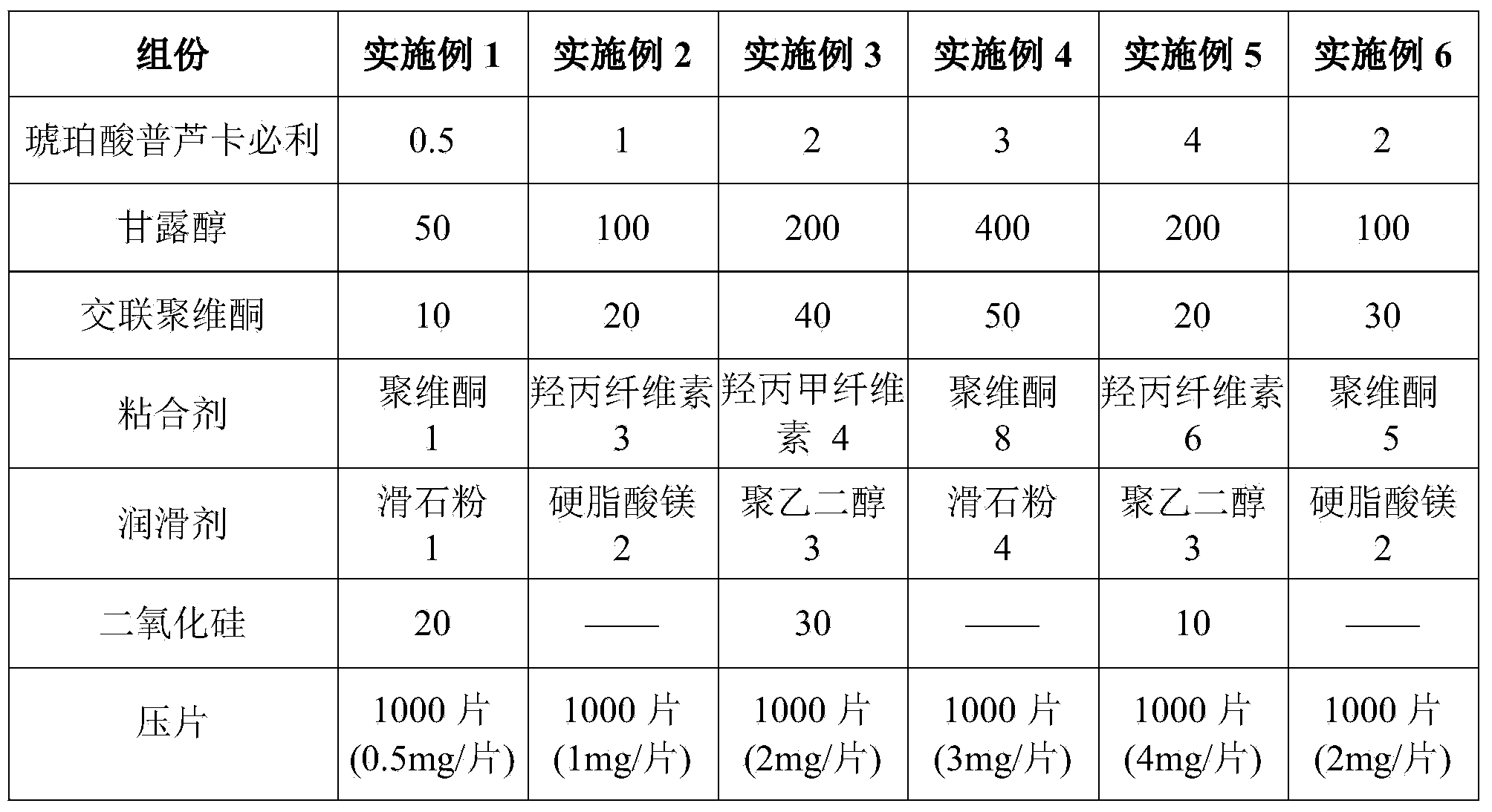
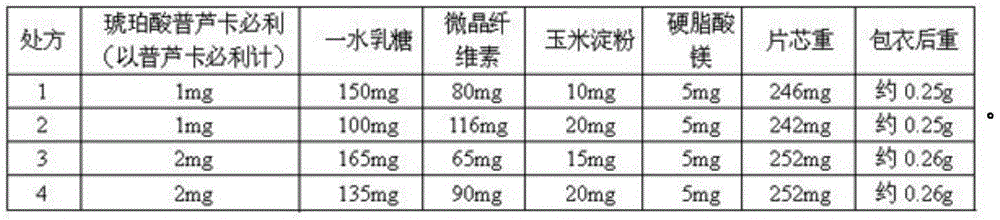
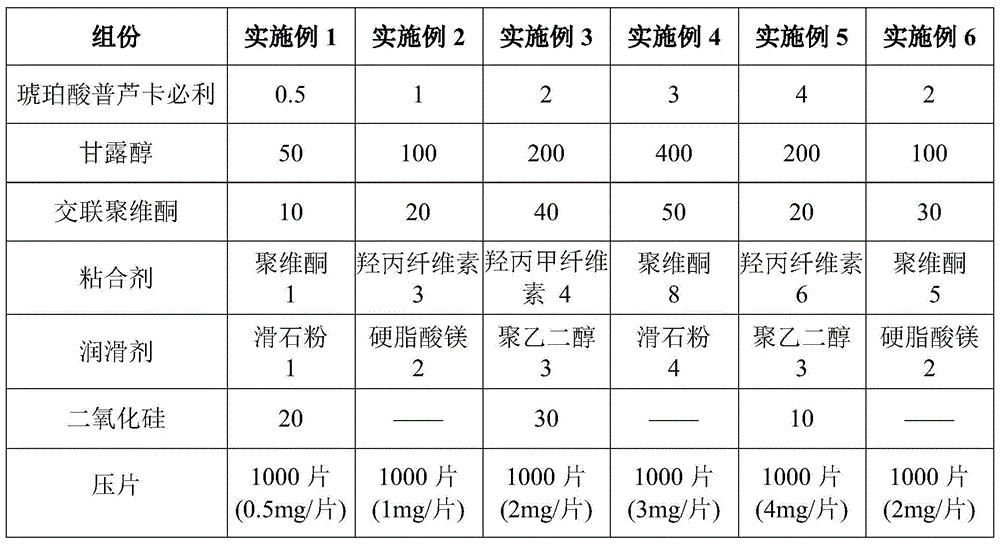
Prucalopride succinate tablet composition
ActiveCN104069080AReasonable prescriptionNo Maillard reactionOrganic active ingredientsDigestive systemCrospovidonesMaillard reaction
The invention provides a prucalopride succinate tablet composition and a preparation method thereof. The prucalopride succinate tablet contains prucalopride succinate, mannitol, crospovidone and the like. The prucalopride succinate tablet has the advantages that a Maillard reaction is not generated; the tablet can be prepared via a wet granulation method; the tablet is high in stability and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Prucalopride succinate pharmaceutical composition free of silicon dioxide and preparation method of prucalopride succinate pharmaceutical composition
ActiveCN104352465AImprove liquidityAddressing Content Uniformity EffectsOrganic active ingredientsDigestive systemAdditive ingredientDiluent
The invention discloses a prucalopride succinate pharmaceutical composition free of a silicon dioxide. The prucalopride succinate pharmaceutical composition comprises the following raw materials in parts by weight: 1-5 parts of prucalopride succinate (based on tiapride prucalopride), 100-280 parts of a diluent, 90-140 parts of a disintegrating agent and 1-10 parts of a lubricating agent. The invention also discloses a preparation method of the pharmaceutical composition. Due to adoption of the composition and the preparation method thereof disclosed by the invention, the specific surface area of the main ingredient prucalopride succinate can be increased; the particle mobility is improved by virtue of a dry granulator production process; the influence on content uniformity caused by a direct pressure technology without the silicon dioxide is solved; the accuracy of the clinical administration dosage is ensured; meanwhile, the pharmaceutical composition can be solved by common production equipment and auxiliary materials; and the industrial production cost is lowered.
Owner:CHENGDU SINO STRONG PHARMA +1
Medicinal composition containing pentoxifylline and prucalopride and medical application thereof
ActiveCN103356630AFully functionalSmall toxicityDigestive systemHeterocyclic compound active ingredientsTreatment effectSphincter
The invention discloses a medicinal composition for treating constipation. According to the medicinal composition, pentoxifylline and prucalopride or medicinally usable salts thereof are used as main active ingredients. The medicinal composition can obviously improve the intestinal transmission function and reduce the pressure of sphincter ani; and an obvious synergistic effect is achieved after the two medicaments are combined. The medicinal composition has clear ingredients, a clear curative effect and a treatment effect of treating both symptoms and root causes of chronic constipation, particularly functional constipation, and thus has a broad medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
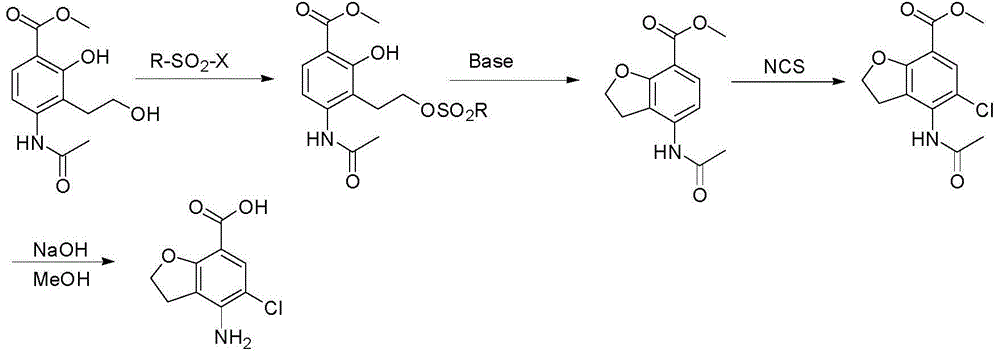
Preparation method of prucalopride intermediate
InactiveCN104529960AReduce usageStarting materials are cheap and readily availableOrganic chemistryBulk chemical productionSodium acetateAluminium chloride
The invention discloses a preparation method of a prucalopride intermediate. The preparation method comprises the following steps: step a, protecting t amino group of a compound I by using trifluoroacetic anhydride to obtain a compound II; step b, under the protection of nitrogen gas, enabling the compound II, chloroacetyl chloride, anhydrous aluminium chloride, nitrobenzene and a halocarbon solvent to react to obtain a compound III; step c, enabling the compound III, an alcohol solvent and sodium acetate to have a reflux reaction to obtain a compound IV; step d, enabling the compound IV, the alcohol solvent, raney nickel and nitrogen with the pressure of 3.0Mpa to react to obtain a compound V; step e, enabling the compound V and N-chloro-succinimide to have a substitution reaction to obtain a compound VI; step f, removing an amino protective group from the compound VI to obtain a compound VII. The method disclosed by the invention has the advantages that initial raw materials are easily available, is simple and convenient to operate, is relatively mild in reaction condition, is capable of avoiding the use of heavy metal reagents and high-toxicity reagents, is high in yield and is quite suitable for large-scale industrial production.
Owner:CHENGDU BAIYU PHARMA CO LTD
Method for preparing prucalopride intermediate
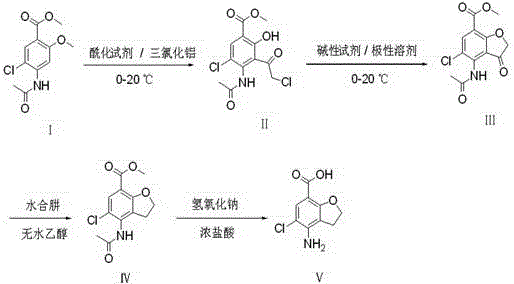
The invention belongs to the field of medicine synthesis, and particularly relates to a method for preparing a prucalopride intermediate. The method comprises the following steps: performing acylation and demethylation reactions on 2-methoxy-4-acetylamino-5-chloro methyl benzoate (I) serving as an initial raw material at 0-20 DEG C under the action of a proper amount of an acylating agent and lewis acid to obtain an intermediate (II); adding an alkaline reagent into the intermediate (II), and performing cyclization reaction at 0-20 DEG C in a polar solvent to obtain an intermediate (III); adding hydrazine hydrate and absolute ethyl alcohol to the intermediate (III), and performing reduction reaction at 70-80 DEG C to obtain an intermediate (IV); performing hydrolysis reaction on the intermediate (IV) at 90-100 DEG C under the action of a sodium hydroxide solution to obtain sodium salt of an intermediate (V), and acidizing with hydrochloric acid to obtain the intermediate (IV). The synthesizing route has mild reaction condition, low production cost and high yield, and is suitable for industrial production.
Owner:阜新峰成化工科技发展有限公司
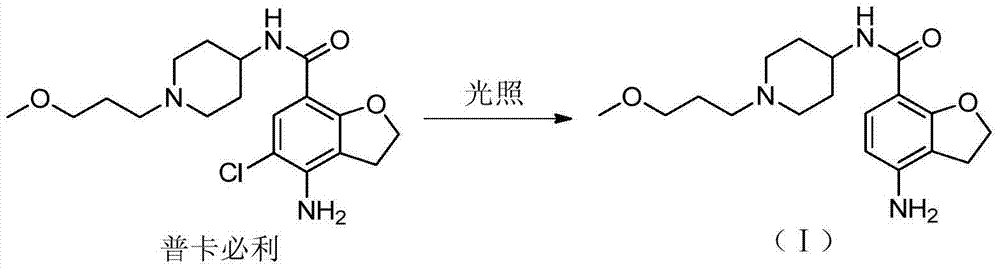
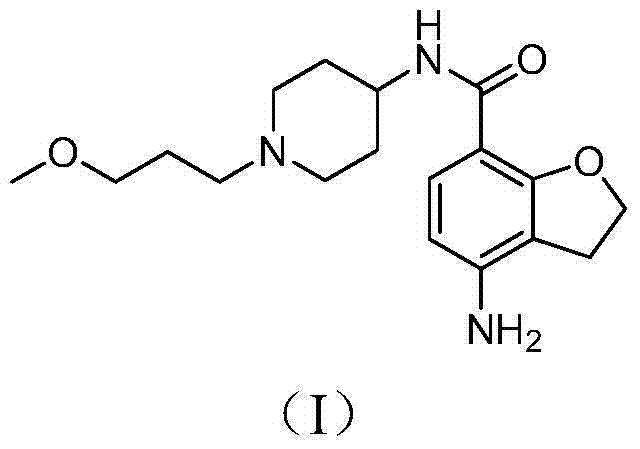
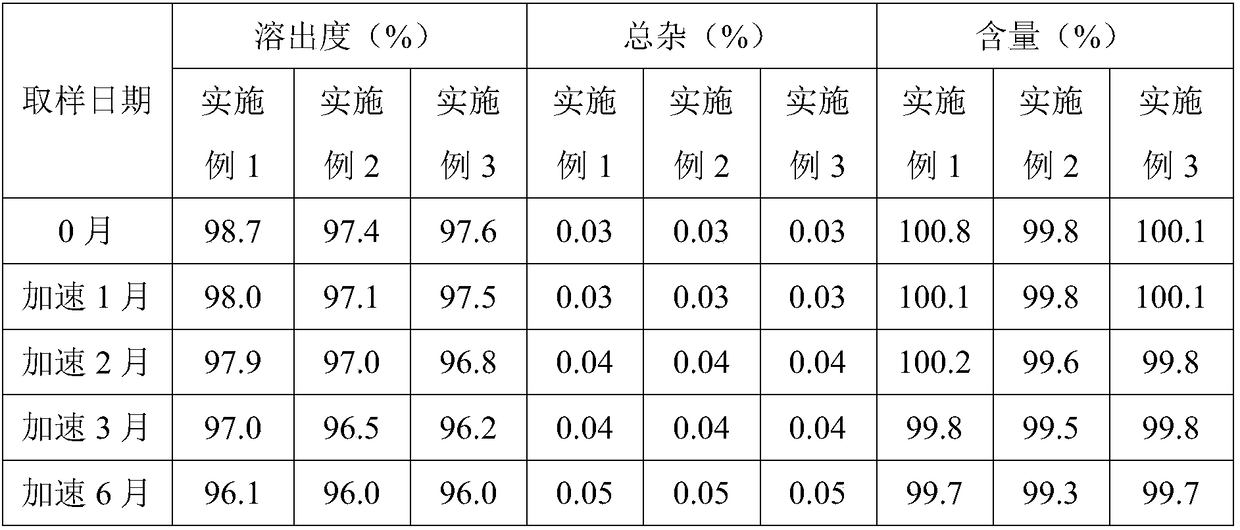
Preparation method for prucalopride degradation impurities
ActiveCN103755689AQuality productionHigh-quality productionOrganic chemistryChemical synthesisHydrogen
The invention relates to a preparation method for prucalopride degradation impurities. Particularly, the invention discloses a synthesis method for degradation impurity compound (I) of drug prucalopride (namely 4-amino-5-chloro-2,3-dihydro-N-[1-(3--methoxypropyl)-4-piperidyl]-7-benzofurancarboxamide succinate) used for treating the constipation of women, and applications in prucalopride research. According to the method, a target compound is obtained by prucalopride through hydrogenation reduction. The compound (I) can be obtained through chemical synthesis for the first time by adopting the method, and the target compound can be obtained through efficient and fast separation.
Owner:连云港恒运药业有限公司
Method for preparing prucalopride impurity
The invention belongs to the field of medicinal chemistry and relates to a method for preparing a prucalopride impurity. The method comprises the following steps: reacting N-[1-(3-methoxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-formamide and a demethoxy reagent so as to obtain the prucalopride impurity N-[1-(3-hydroxypropyl)]-4-amino-5-chloro-2,3-dihydrobenzofuran-7-formamide. The adopted reagent is economic and readily available, the synthetic method is simple and convenient in operation, and the reaction conditions are mild.
Owner:万全万特制药江苏有限公司
Therapy for enteric infections
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhoea, chronic idiopathic nausea, IBD-associated constipation and diarrhoea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers diarrhoea, chronic idiopathic nausea, IBD-associated constipation and diarrhoea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinsons disease, MS, Alzheimers Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Prucalopride sccinate tablet and preparation method thereof
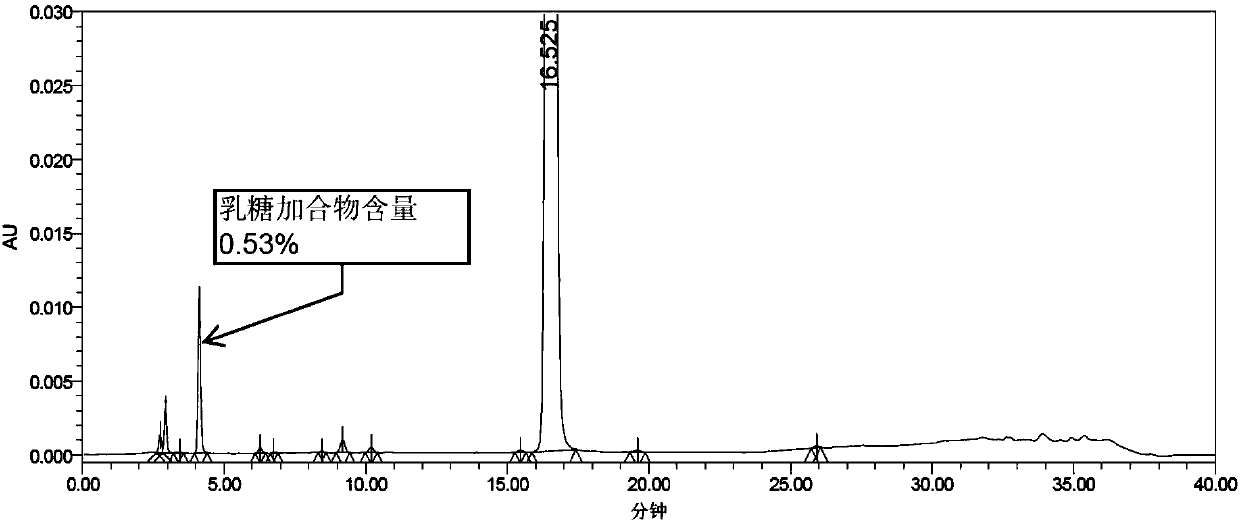
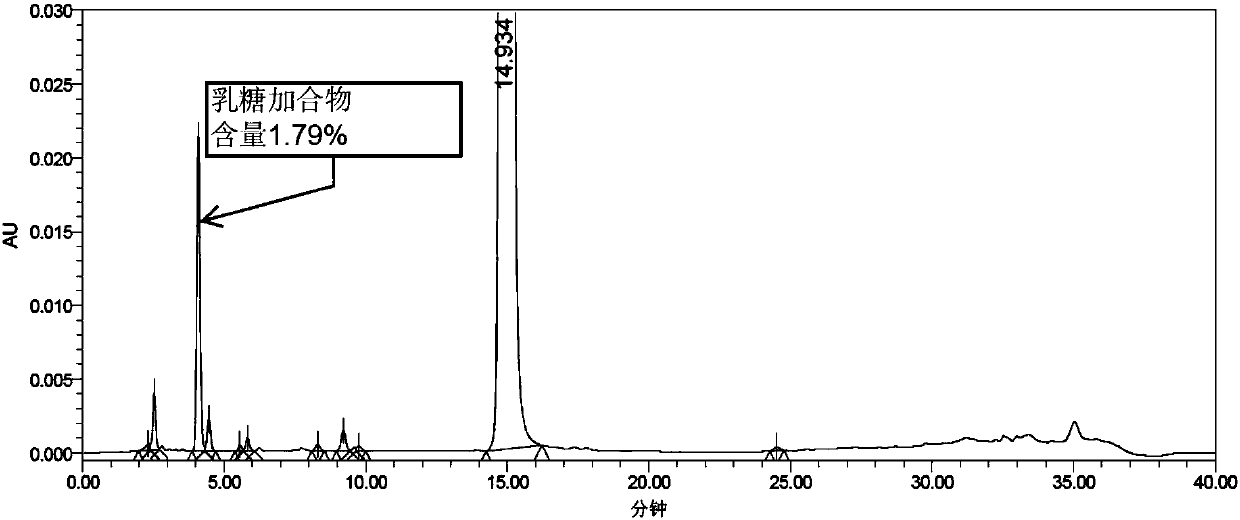
InactiveCN107582535ASolve instabilityEasy to operateOrganic active ingredientsDigestive systemSpray driedLactose
The invention provides a prucalopride sccinate tablet and a preparation method thereof. The prucalopride sccinate tablet comprises prucalopride sccinate, with the weight of 0.5 to 2.0 percent of the total weight of the preparation, particle lactose or spray-dried lactose, with the weight of 65 to 94 percent of the total weight of the preparation, microcrystalline cellulose and / or a compound thereof, with the weight of 5 to 30 percent of the total weight of the preparation, and a lubricant, with the weight of 0.5 to 3 percent of the total weight of the preparation. The method adopts a direct powder mixing tabletting method, and the preparation technology comprises the following steps: (1), uniformly mixing the prucalopride sccinate, lactose and microcrystalline cellulose, so as to obtain amixture I, and (2), adding the lubricant to the mixture I, totally blending, tabletting, coating and packaging.
Owner:SHIJIAZHUANG NO 4 PHARMA
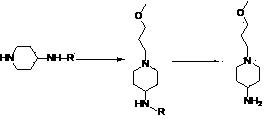
A kind of synthetic method of n-(3-methoxypropyl)-4-aminopiperidine
The present invention relates to a method for synthesizing a prucalopride key intermediate, and in particular, to a method for synthesizing N-(3-methoxylpropyl)-4-aminopiperidine, comprising reacting 1-(3-methoxylpropyl)piperidine-4-one with benzylamine substituted with a benzene ring to generate 1-(3-methoxylpropyl)-4-benzylaminopiperidine under the action of a reductant, and obtaining N-(3-methoxylpropyl)-4-aminopiperidine via catalysis of palladium-carbon.
Owner:YOUCARE PHARMA GROUP +1
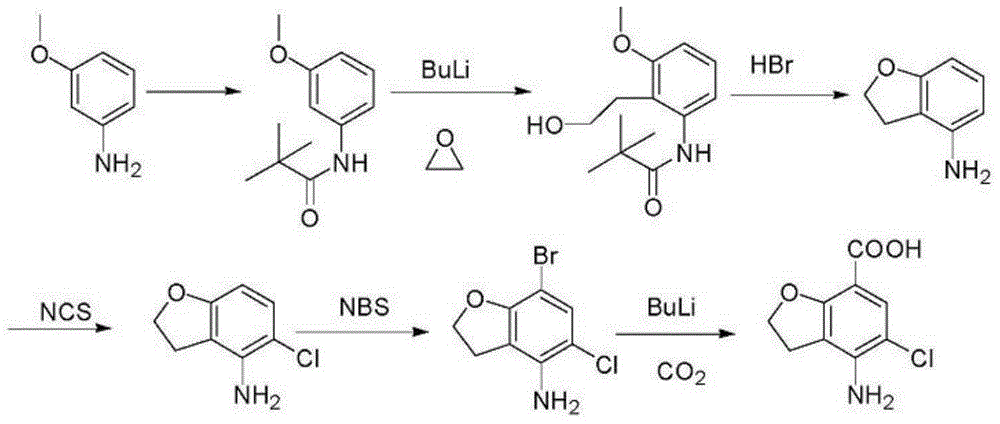
Synthetic method for 4-amino-5-chloro-2,3-dihydro-7-benzofurancarboxylic acid
The present invention relates to a drug intermediate synthetic method, in particular to a synthetic method for 4-amino-5-chloro-2,3-dihydro-7-benzofurancarboxylic acid of Prucalopride Succinate intermediate. The method comprises performing hydrolysis adopting a two-step method. According to the synthetic method disclosed by the invention, the raw material can be reacted adequately; the reaction time can be shortened; the obtained product is high in purity; and the yield is improved.
Owner:SHANGHAI FAMO BIOTECH CO LTD
Prucalopride succinate tablet composition and preparation method thereof
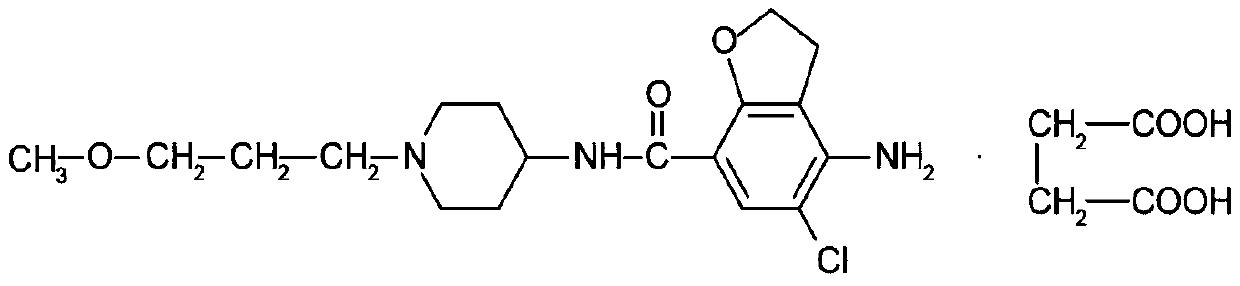
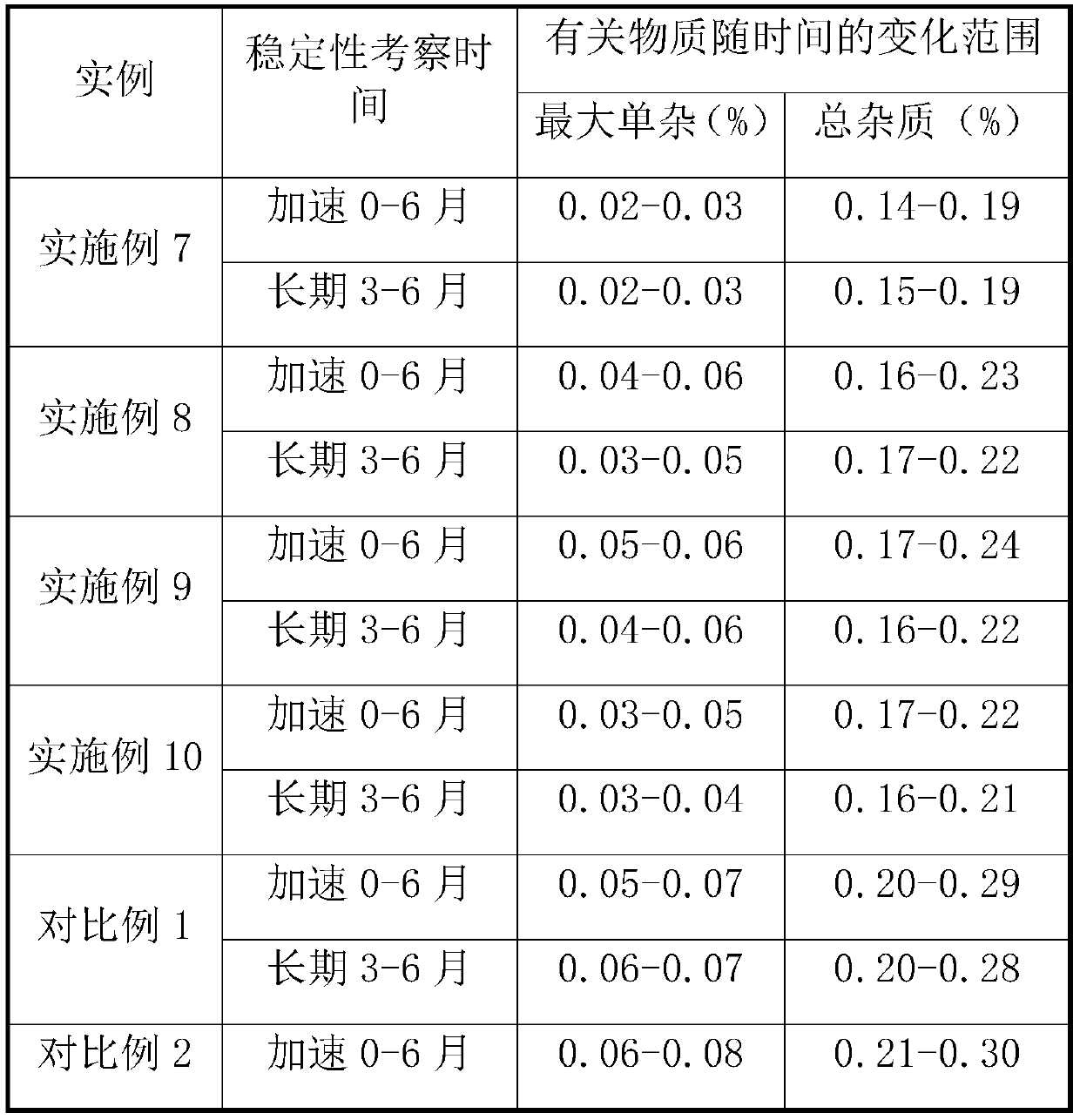
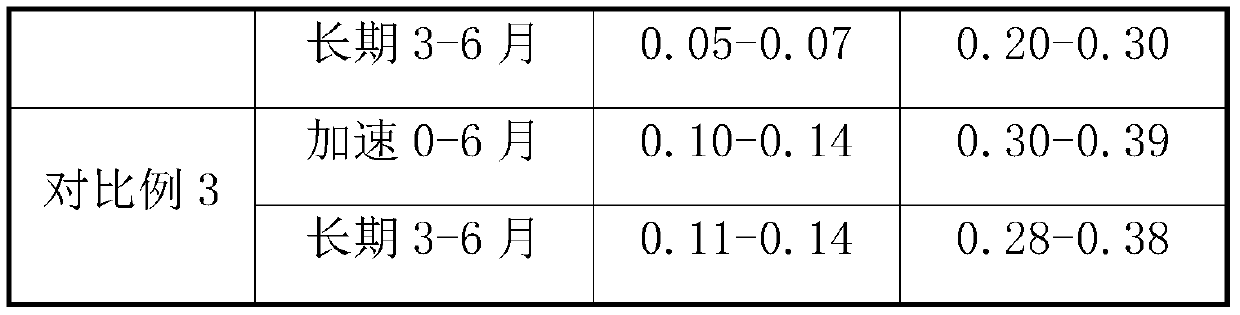
PendingCN111184695ASimple processImprove stabilityOrganic active ingredientsDigestive systemSuccinic acidBiological availability
The invention relates to a prucalopride succinate tablet composition and a preparation method thereof, and provides a prucalopride succinate tablet composition which is non-toxic, good in stability, high in uniformity and biological availability, simple in technology and low in cost, and a preparation method of the prucalopride succinate tablet composition.
Owner:JIANGSU HANSOH PHARMA CO LTD
Novel enteric combination therapy
There is disclosed herein a composition for treating gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers' diarrhoea, chronic idiopathic nausea, IBD-associated constipation and diarrhoea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinson's disease, MS, Alzheimer's Disease, Motor Neurone Disease or autism, the composition comprising: (i) at least two anti-Clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (ii) at least one anti-Clostridial agent selected from the above combined with an opioid blocking agent. There is also disclosed herein a method of treating various gastrointestinal or neurological disorders, constipation, functional constipation, irritable bowel syndrome, diverticulitis, travelers' diarrhoea, chronic idiopathic nausea, IBD-associated constipation and diarrhoea, pseudo-obstruction, diabetic gastroparesis, cyclic vomiting, reflux oesophagitis, autism enteropathy, flatulence, halitosis, chronic fatigue, bloating, proctalgia fugax, Parkinson's disease, MS, Alzheimer's Disease, Motor Neurone Disease or autism, the method comprising administering orally, via enema or by suppository: (i) a composition of the invention; (ii) at least two anti-Clostridial agents selected from the group consisting of: vancomycin, vancomycin derivatives, a multi-valent polymer of vancomycin, aminoglycosides, nitroimidazoles, ansamysins, nifuroxazide, colchicine, prucalopride, prokinetic agent and 5-aminosalicylic acid; or (iii) at least one anti-Clostridial agent selected from the above and an opioid blocking agent to a patient in need of such treatment.
Owner:BORODY THOMAS JULIUS
Prucalopride oral solution
The present invention is concerned with an oral aqueous solution comprising prucalopride or pharmaceutically acceptable acid addition salts thereof having good organoleptic properties.
Owner:SHIRE MOVETIS
Prucalopride succinate capsule and preparation method thereof
InactiveCN108175757AReasonable prescriptionDisintegrates quicklyOrganic active ingredientsDigestive systemMedicineMedical prescription
The invention provides a prucalopride succinate capsule and a preparation method thereof. The capsule is prepared from substances in parts by weight as follows: 0.5-2 parts of prucalopride succinate,70-80 parts of filler, 10-20 parts of a disintegrant, 4-8 parts of a glidant and 2-5 parts of a lubricant. Raw and auxiliary materials are pretreated and mixed, a capsule shell is filled directly withthe mixture finally, and the capsule is prepared. The formula is reasonable in design, the preparation process is simple, and the product is stable and reliable in quality and high in bioavailabilityand has great market development prospect.
Owner:PEKING UNIV FOUNDER GRP CO LTD +3
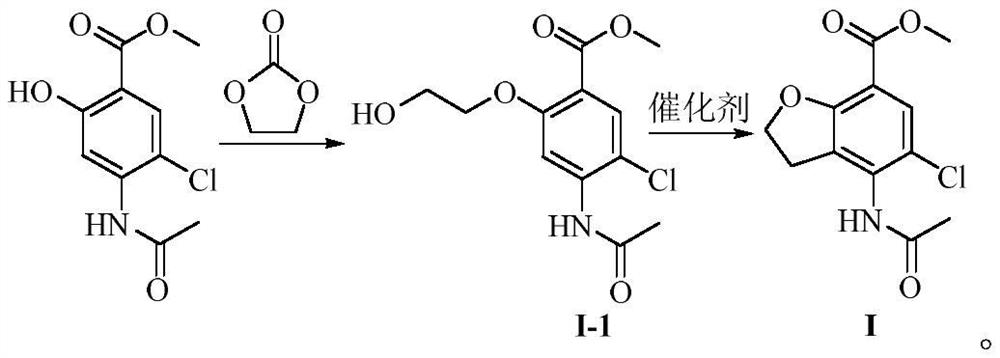
Preparation method of 4-acetamido-5-chloro-2, 3-dihydrobenzofuran-7-carboxylic acid methyl ester
PendingCN114591278AHigh yieldHigh purityOrganic chemistryBulk chemical productionMetaclazepamDrugs synthesis
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of a prucalopride intermediate 4-acetamido-5-chloro-2, 3-dihydrobenzofuran-7-carboxylic acid methyl ester. According to the method, 4-acetamido-5-methyl chlorosalicylate is taken as a reaction raw material, ethoxyl is introduced by ethylene carbonate, then a benzotetrahydrofuran ring is directly constructed through an intramolecular alkylation reaction, a compound I is prepared, and the reaction equation is shown in the specification. According to the preparation method, the reaction route can be effectively shortened, the operation is safe, simple and convenient, the prepared target product is high in yield and purity, and the preparation method is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of prucalopride succinate tablet composition
ActiveCN104069080BReasonable prescriptionNo Maillard reactionOrganic active ingredientsDigestive systemCrospovidonesMaillard reaction
The invention provides a prucalopride succinate tablet composition and a preparation method thereof. The prucalopride succinate tablet contains prucalopride succinate, mannitol, crospovidone and the like. The prucalopride succinate tablet has the advantages that a Maillard reaction is not generated; the tablet can be prepared via a wet granulation method; the tablet is high in stability and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com