Method for preparing prucalopride intermediate
A technology for prucalopride and intermediates, which is applied in the field of preparing prucalopride intermediates, can solve the problems of safety, high synthesis cost, and high price, and achieve the goal of having many manufacturers, low raw material prices, and expanding the scale of industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
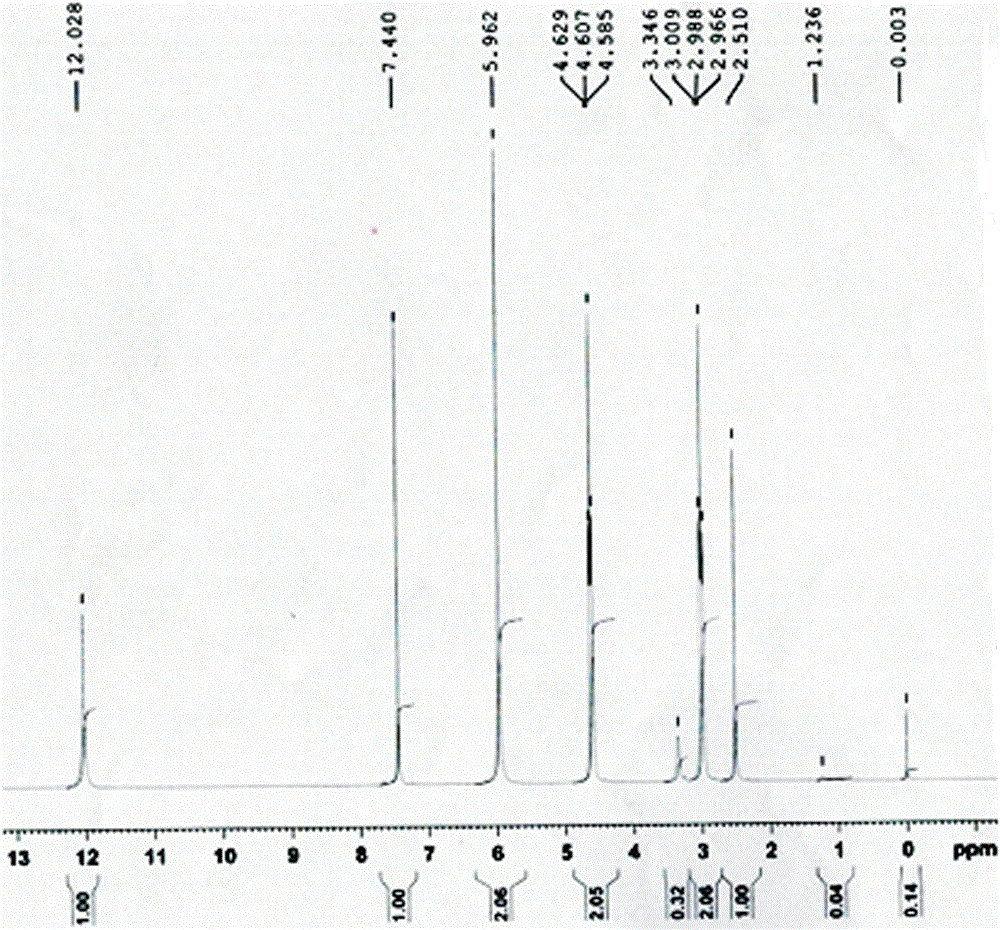
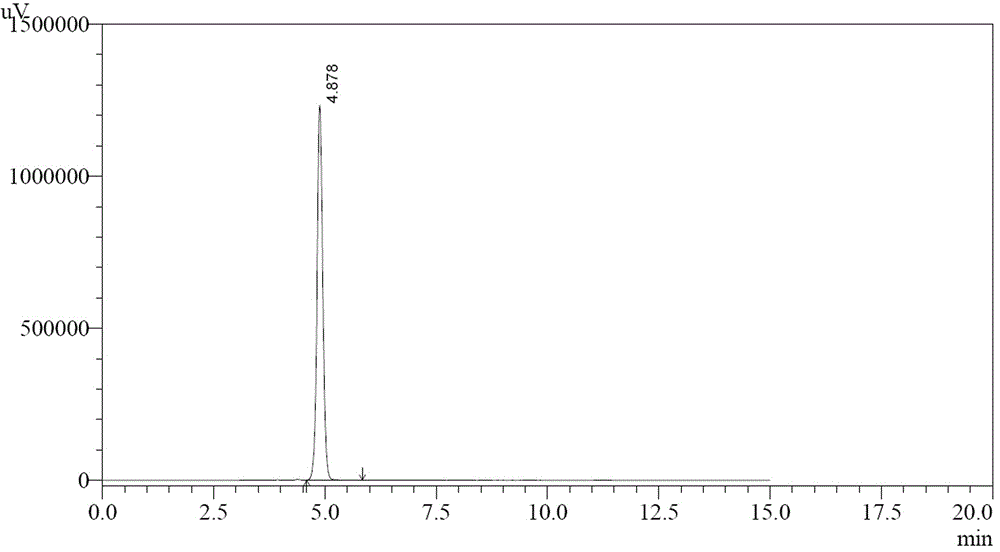
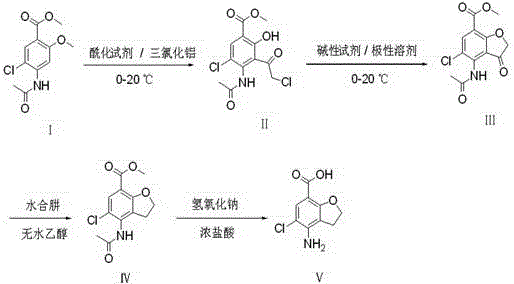
[0023] Add 77 g of aluminum trichloride to 500 mL of dichloromethane, cool to 0-10 °C, add 26 g of chloroacetyl chloride dropwise, control the temperature at 0-10 °C, stir for 0.5 h, and the stirring speed is 300 r / min. Add the dichloromethane solution (50g+500mL dichloromethane) of the starting material I dropwise to the reaction system, the temperature is 0-10°C, keep stirring for 4 hours, and the stirring speed is 300r / min; add 500mL6% hydrochloric acid solution dropwise, control When the temperature is lower than 20°C, use a separatory funnel to separate the dichloromethane from the organic layer, and concentrate the organic layer under reduced pressure until no dichloromethane is evaporated (the temperature is not higher than 60°C) to obtain an oil, and add 50 mL of anhydrous Ethanol and 450mL water were beaten and dispersed at 0-10°C for 1h, suction filtered, and air-dried at 50-60°C for 8h to obtain intermediate (II), 52g of light gray solid, with a yield of 83%.
[002...
Embodiment 2
[0028] Add 128g of aluminum trichloride to 1000mL of dichloromethane, cool to 0-10°C, add 52g of chloroacetyl chloride dropwise, control the temperature at 0-10°C, stir for 0.5h, and the stirring speed is 300r / min. Add the dichloromethane solution of starting material I (100g + 1000mL dichloromethane) dropwise to the reaction system, control the temperature at 0-10°C, and keep stirring for 4h after the addition. Add 1000mL of 6% hydrochloric acid solution dropwise, control the temperature below 20°C, separate the organic layer with dichloromethane with a separatory funnel, and concentrate the organic layer under reduced pressure until no dichloromethane evaporates (temperature is not higher than 60°C) to obtain an oily substance , adding 100mL ethanol and 900mL water to the oil, beating and dispersing at 0-10°C for 1h, suction filtration, and drying to obtain intermediate (II), 92g of light gray solid, yield 73%.
[0029] Add 75g of intermediate (II) to 450mL of N,N-dimethylfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com