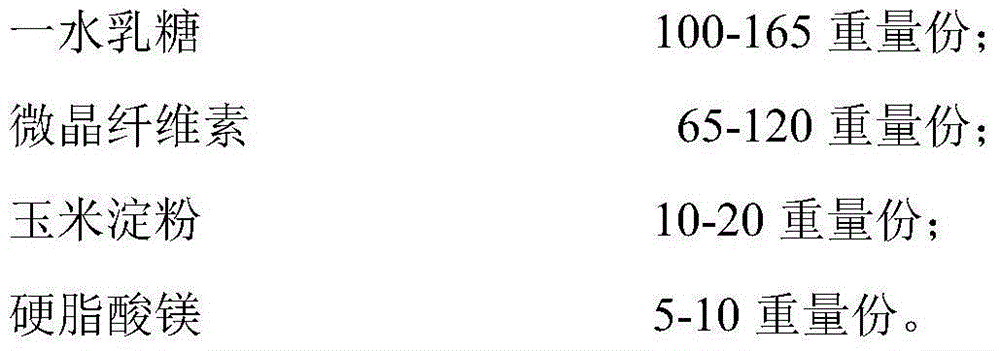Prucalopride succinate pharmaceutical composition free of silicon dioxide and preparation method of prucalopride succinate pharmaceutical composition
A technology of prucalopride and silicon dioxide, which can be used in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulations, etc., can solve the problems of hidden safety hazards, high prices, and very high fluidity requirements, and can achieve guaranteed Accuracy, reduced production costs, improved particle flowability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
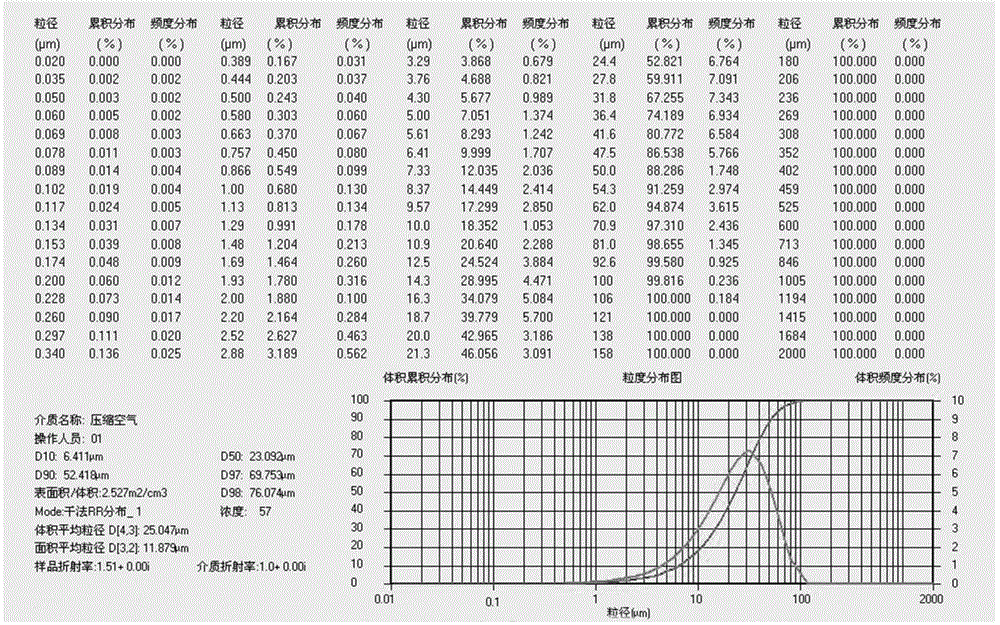
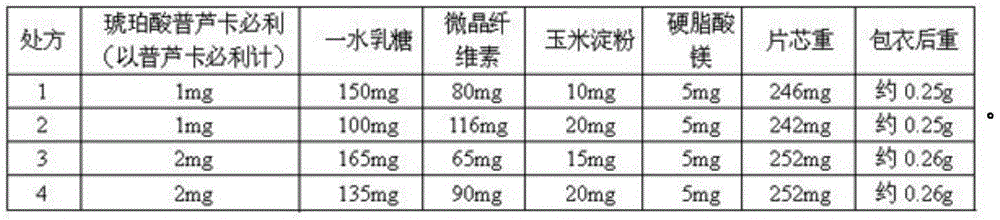
[0041] Weigh the raw materials according to formula 1, carry out jet milling of prucalopride succinate, collect the materials, and measure the particle size distribution (dry method measurement), requiring D10=0.1~8um, D50=10~30um, D90<60um. Then, the lactose monohydrate, the microcrystalline cellulose, and the corn starch are pulverized to control the particle size distribution D90<75um respectively. Using the doubling method, first mix prucalopride succinate and lactose monohydrate with particle size distribution meeting the requirements, and mix each time for 2 to 5 minutes; mix prucalopride succinate with lactose monohydrate After completion, mix with 50% of the prescription amount of microcrystalline cellulose for 2 to 5 minutes, then add the remaining microcrystalline cellulose and cornstarch, and mix for 10 to 20 minutes. After mixing, take 10 samples at different positions of the mixer to measure the content, and the RSD value is required to be less than 1.0%. Put the...
Embodiment 2-4
[0043] Example 2-4 Formula 2-4 was used and prepared according to the method of Example 1.
Embodiment 5
[0045] Particle content uniformity detection. The detection method is UV method; the detection wavelength is 274nm, the solvent is 0.001mol / L hydrochloric acid solution, and the measured concentration is 10ug / ml.
[0046] sample number Prescription 1 Prescription 2 Prescription 3 4 prescriptions 1# 0.414% 0.423% 0.811% 0.812% 2# 0.415% 0.422% 0.813% 0.814% 3# 0.416% 0.424% 0.814% 0.811% 4# 0.415% 0.425% 0.815% 0.809% 5# 0.414% 0.423% 0.811% 0.808% 6# 0.415% 0.422% 0.810% 0.816% 7# 0.415% 0.424% 0.810% 0.812% 8# 0.413% 0.423% 0.811% 0.811% 9# 0.416% 0.426% 0.812% 0.810% 10# 0.415% 0.421% 0.815% 0.813% RSD 0.22%<1.0% 0.35%<1.0% 0.24%<1.0% 0.29%<1.0%
[0047] Table 2 Determination data of tablet content uniformity
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com