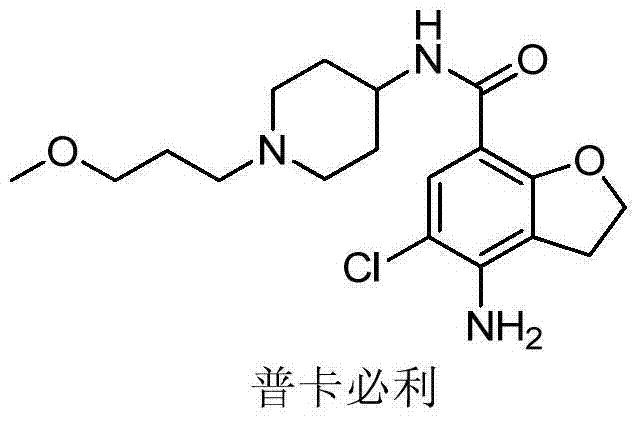
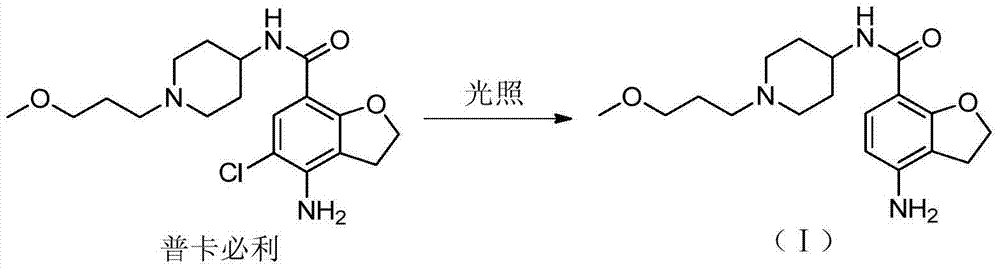
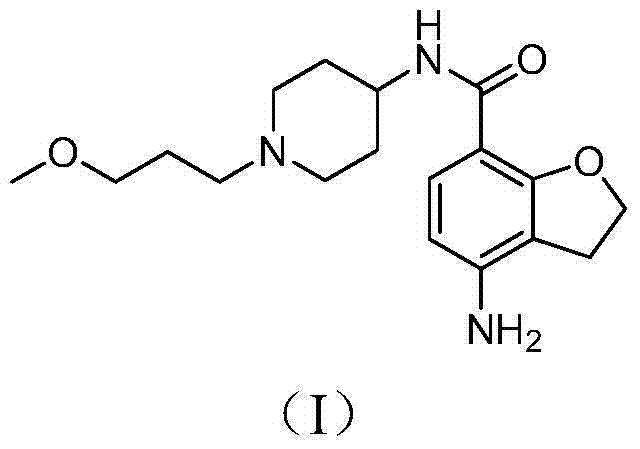
Preparation method for prucalopride degradation impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add prucalopride (1.5g) into the reaction flask, add methanol (150ml), then add 10% palladium carbon (2.0g), sodium hydroxide (1.0g), and hydrogenate at 10-30°C under normal pressure 3 days. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain Compound I (1.3 g), with a molar yield of 96%.
[0030] MS-ESI(m / z):334.33[M+H] + .
[0031] The structure was determined to be compound I by structural analysis.
Embodiment 2
[0033] Add prucalopride (1.5g) into the reaction flask, add isopropanol (150ml), then add 10% palladium carbon (2.0g), sodium hydroxide (1.0g), and add at 10-30°C under normal pressure Hydrogen reaction for 3 days. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain Compound I (1.2 g), with a molar yield of 88%.
[0034] MS-ESI(m / z):334.33[M+H] + .
[0035] The structure was determined to be compound I by structural analysis.
Embodiment 3
[0037] Add prucalopride (1.5g) into the reaction flask, add methanol (150ml), then add 10% palladium carbon (2.0g), potassium hydroxide (1.1g), hydrogenation reaction at 10-30°C under normal pressure 3 days. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain Compound I (1.2 g), with a molar yield of 88%.
[0038] MS-ESI(m / z):334.33[M+H] + .
[0039] The structure was determined to be compound I by structural analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com