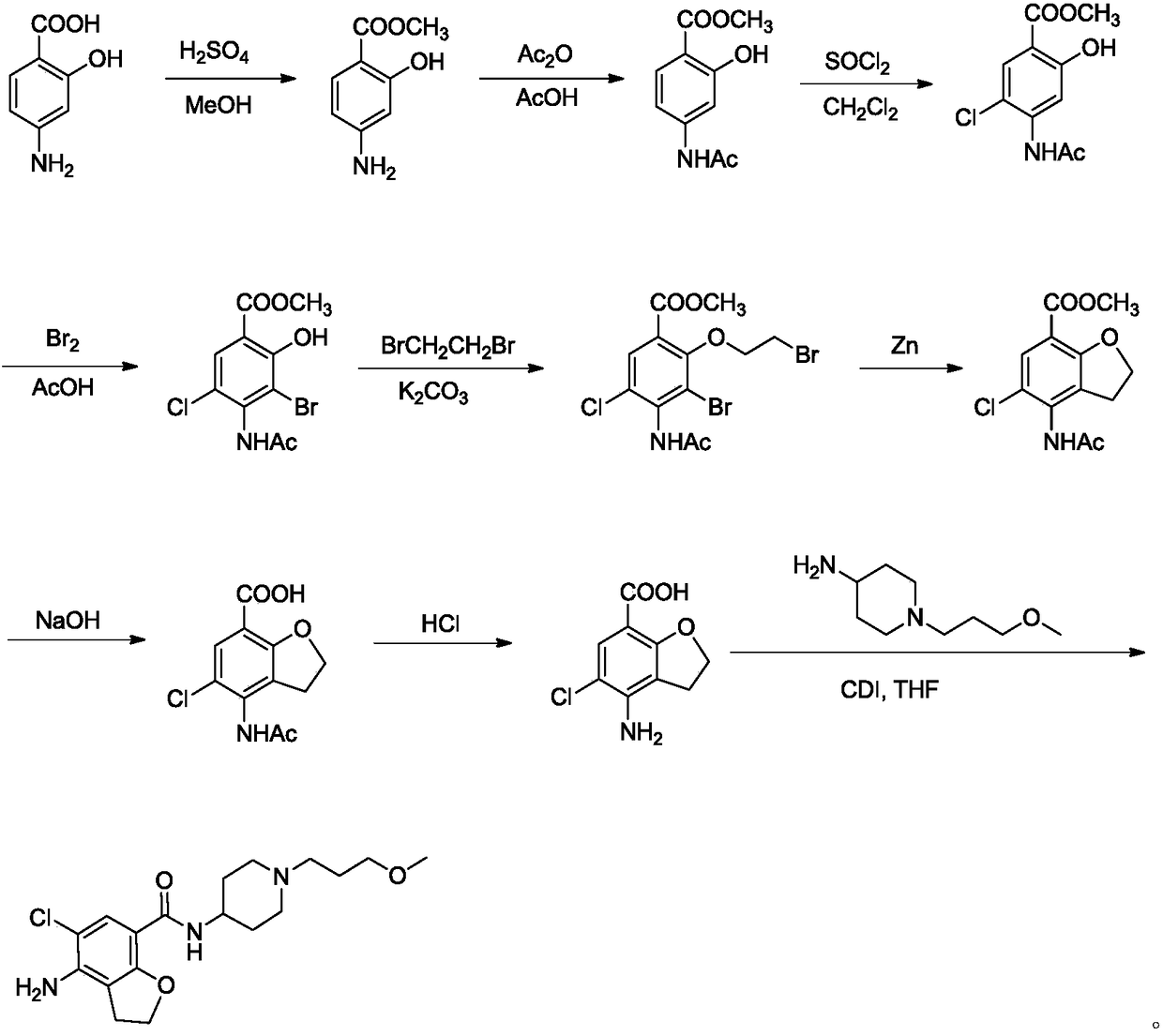
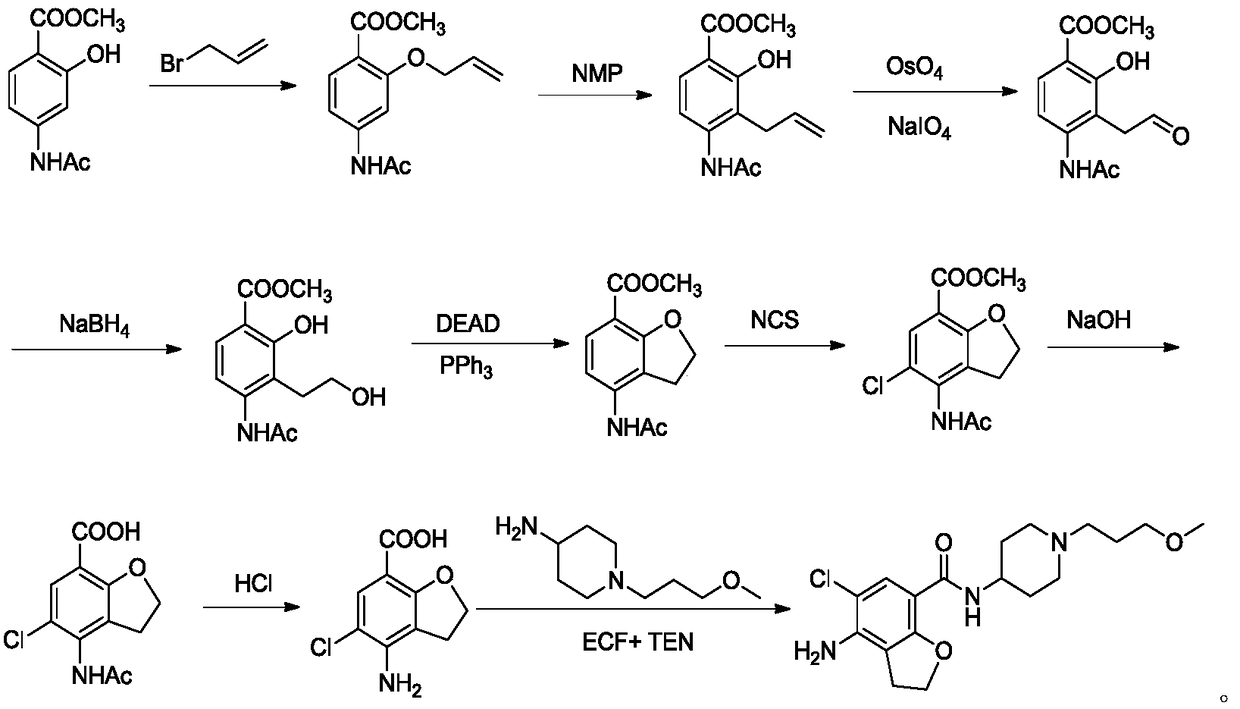
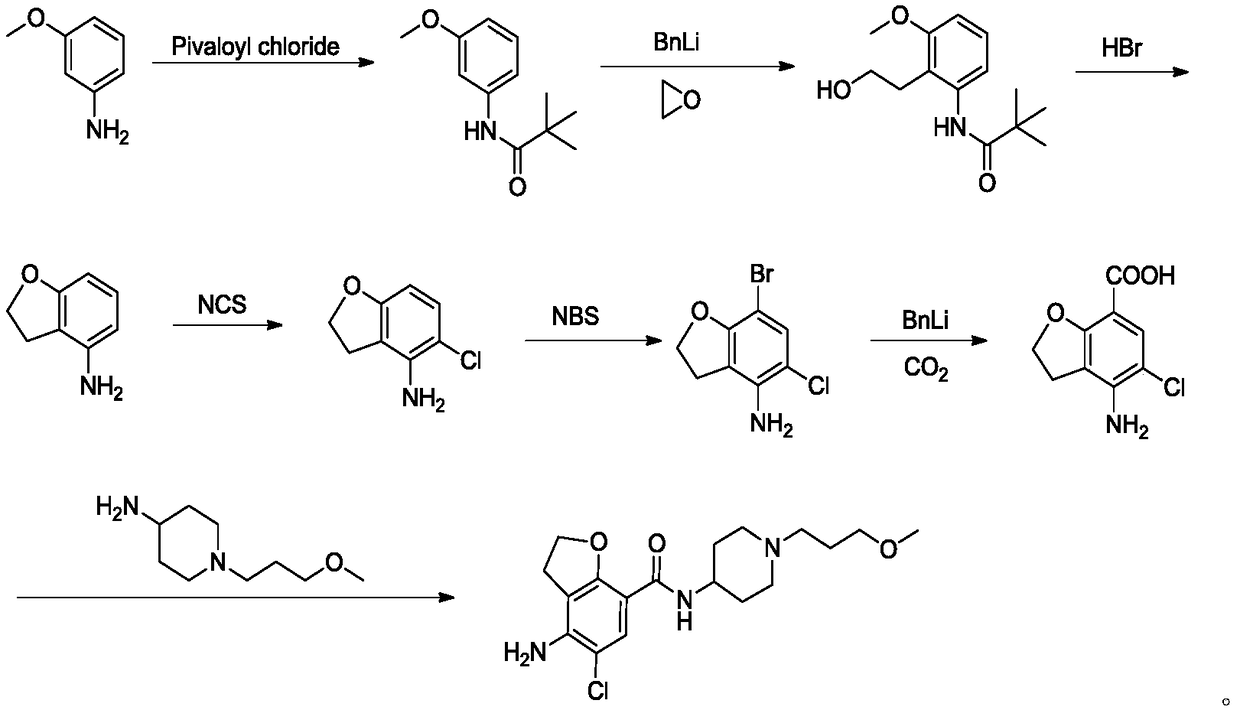
Preparation method of prucalopride succinate
A technology of reaction and dioxolane, which is applied in the field of preparation of prucalopride, can solve problems such as inconvenient operation, and achieve the effects of simple and convenient operation, high product purity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step 1, the preparation of 2-(4-nitrophenyl)-1,3-dioxolane: take 151g of 4-nitrobenzaldehyde, 62g of ethylene glycol, and 190g of p-toluenesulfonic acid, and dissolve them in 300mL of toluene , after heating to reflux for 12 hours, the toluene was recovered under reduced pressure, the residue was added to 300 mL of ethyl acetate, washed with water, the organic layer was dried over anhydrous sodium sulfate, and the filtrate was filtered and distilled under reduced pressure to obtain 180 g of a light yellow solid.
[0046] Step 2, the preparation of 2-(4-aminophenyl)-1,3-dioxolane: Take 195g of 2-(4-nitrophenyl)-1,3-dioxolane, dissolve it in 400mL In water ethanol, add palladium carbon catalyst (Pd / C) 10g, feed hydrogen, after heating to reflux reaction for 12h, cool to room temperature, filter to remove palladium carbon catalyst (Pd / C), filtrate decompression recovery solvent white solid 165g.
[0047] Step 3, the preparation of 2-(4-acetylaminophenyl)-1,3-dioxolane: Tak...
Embodiment 2
[0055] Step 1, the preparation of 2-(4-nitrophenyl)-1,3-dioxolane: take 151g of 4-nitrobenzaldehyde, 70g of ethylene glycol, and 200g of p-toluenesulfonic acid, dissolve in 300mL of toluene , after heating to reflux for 12 hours, the toluene was recovered under reduced pressure, the residue was added to 300 mL of ethyl acetate, washed with water, the organic layer was dried over anhydrous sodium sulfate, and the filtrate was filtered and distilled under reduced pressure to obtain 191 g of a light yellow solid.
[0056] Step 2, the preparation of 2-(4-aminophenyl)-1,3-dioxolane: Take 195g of 2-(4-nitrophenyl)-1,3-dioxolane, dissolve it in 400mL In water ethanol, add palladium carbon catalyst (Pd / C) 10g, feed hydrogen, after heating to reflux reaction for 12h, cool to room temperature, filter to remove palladium carbon catalyst (Pd / C), filtrate decompression recovery solvent white solid 165g.
[0057] Step 3, the preparation of 2-(4-acetylaminophenyl)-1,3-dioxolane: Take 165g of...
Embodiment 3
[0065] Step 1, the preparation of 2-(4-nitrophenyl)-1,3-dioxolane: take 151g of 4-nitrobenzaldehyde, 75g of ethylene glycol, and 210g of p-toluenesulfonic acid, and dissolve them in 300mL of toluene , after heating to reflux for 12 hours, the toluene was recovered under reduced pressure, the residue was added to 300 mL of ethyl acetate, washed with water, the organic layer was dried over anhydrous sodium sulfate, and the filtrate was filtered and distilled under reduced pressure to obtain 195 g of a light yellow solid.
[0066] Step 2, the preparation of 2-(4-aminophenyl)-1,3-dioxolane: Take 195g of 2-(4-nitrophenyl)-1,3-dioxolane, dissolve it in 400mL In water ethanol, add palladium carbon catalyst (Pd / C) 10g, feed hydrogen, after heating to reflux reaction for 12h, cool to room temperature, filter to remove palladium carbon catalyst (Pd / C), filtrate decompression recovery solvent white solid 165g.
[0067] Step 3, the preparation of 2-(4-acetylaminophenyl)-1,3-dioxolane: Tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com