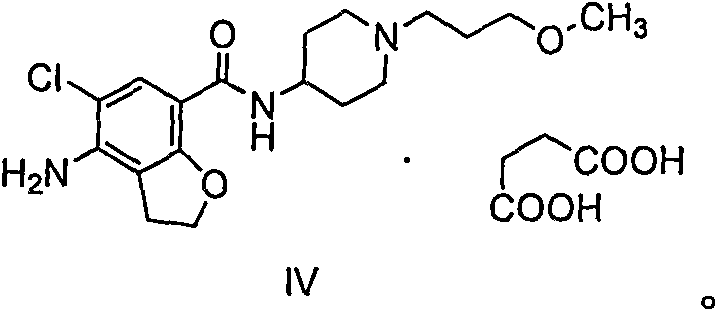
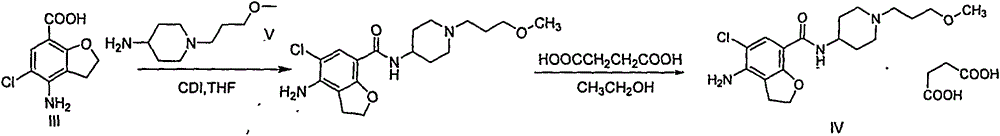
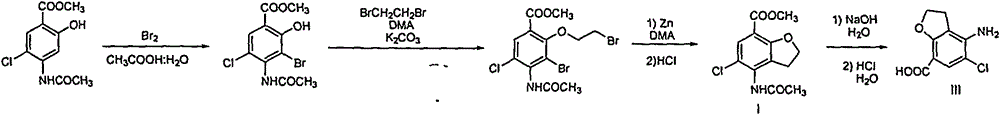
Synthetic method for 4-amino-5-chloro-2,3-dihydro-7-benzofurancarboxylic acid
A synthetic method, the technology of propylene glycol monomethyl ether, applied in the direction of organic chemistry, etc., can solve the problems of incomplete reaction of raw materials, long time, poor purity of compound I, etc., and achieve the effects of shortening the reaction time, increasing the yield, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 4-acetylamino-5-chloro-2,3-dihydro-7-benzofurancarboxylic acid (compound of formula II)
[0037] Formula I compound (100g, purity 80%) obtained above in the mixed solvent of water (400ml) and propylene glycol monomethyl ether (80ml), add 40% sodium hydroxide aqueous solution (100g), react at 70 ℃ for 2 hours , TLC monitors that the reaction is complete. The temperature of the reaction solution was lowered, filtered with suction, the filter cake was washed once with water, and dried to obtain the sodium salt of the compound of formula II (63 g), with a purity of 98.2%.
[0038] Salts of the compound of formula II such as potassium salt, lithium salt and ammonium salt can be synthesized by a method similar to Example 1.
[0039] MS(m / z): 256.1[M+H] +
[0040] 1 H-NMR (DMSO-d6, 400MHz)
[0041] δ: 2.07(3H, s), 3.05(2H, t), 4.65(2H, t), 7.61(1H, s), 9.89(1H, s), 12.89(1H, s)
Embodiment 2
[0043] Preparation of 4-amino-5-chloro-2,3-dihydro-7-benzofurancarboxylic acid (compound of formula III)
[0044] The sodium salt of the compound of formula II (63g, 0.227mol) was added to a reaction flask, 3mol / L NaOH aqueous solution (250ml) and propylene glycol monomethyl ether (40ml), and reacted at 100° C. for 3 hours, and TLC monitored that the reaction was complete. Cool down to 35°C, filter with suction, wash the filter cake with water, put the obtained filter cake into a reaction flask, add water (500ml) and propylene glycol monomethyl ether (50ml), heat to 85°C, heat filter, add 6mol / L hydrochloric acid aqueous solution dropwise to the filtrate to adjust When the pH reached 2, a large amount of solids were precipitated, cooled, filtered with suction, washed the filter cake with water, and dried to obtain the compound of formula III (47 g), yield: 97%, purity: 99.5%.
[0045] MS(m / z): 214.6[M+H] +
[0046] 1 H-NMR (DMSO-d6, 400MHz)
[0047] δ: 2.97 (2H, t), 4.60 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com