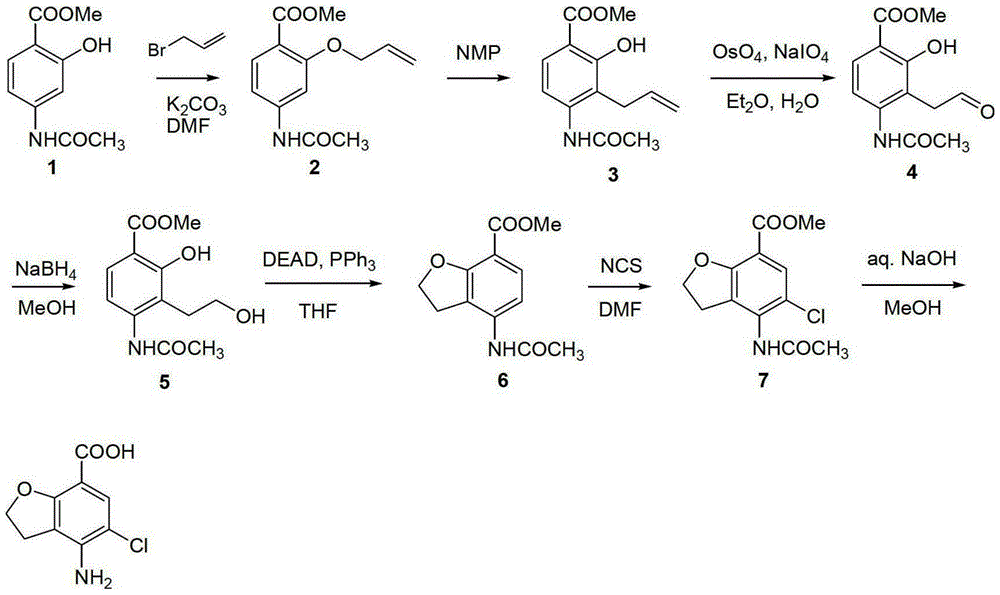
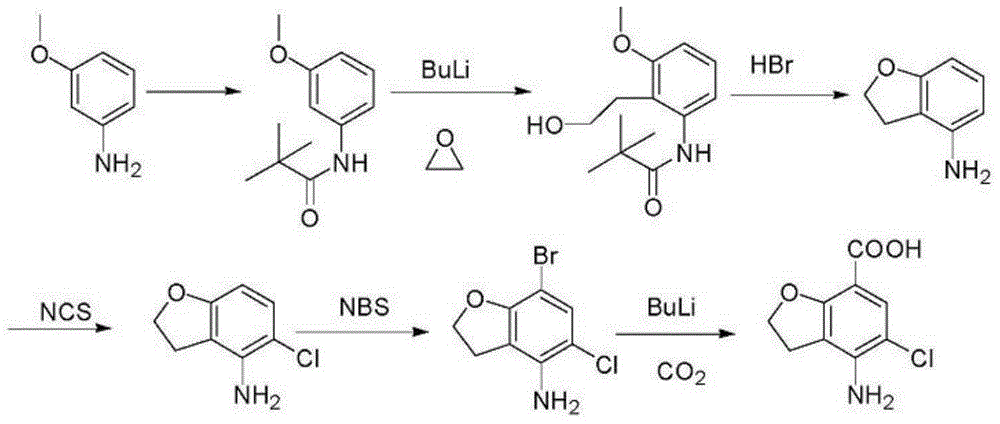
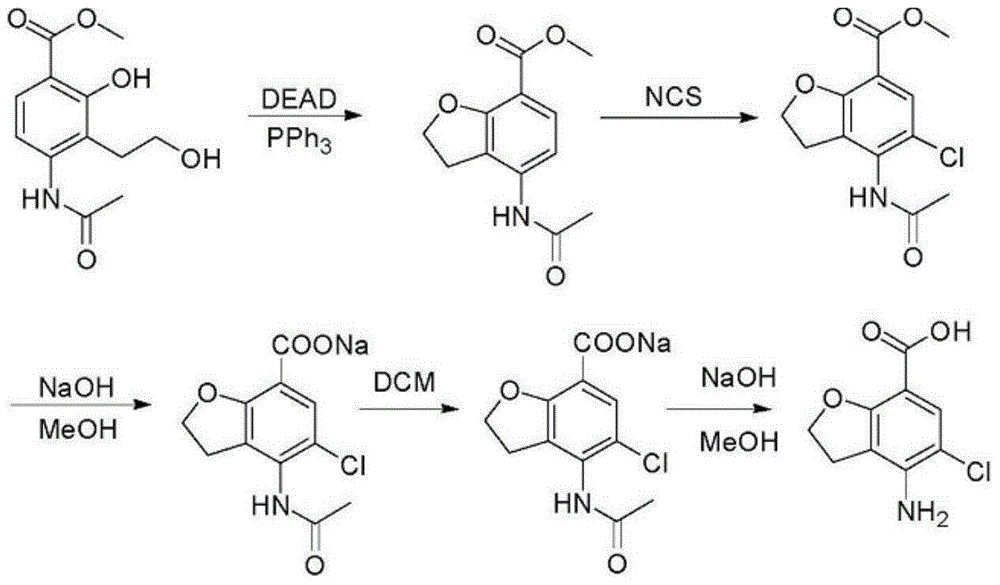
Preparation method of prucalopride intermediate
A technology for prucalopride and intermediates, which is applied in the field of preparation of prucalopride intermediates, can solve problems such as unsuitable for industrial scale production, difficult to obtain, cumbersome steps, etc., and avoid heavy metal reagents and highly toxic reagents , suitable for large-scale industrial production, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 1. Preparation of Compound II
[0065] Compound I (167.1g, 1mol), triethylamine (111.1g, 1.1mol) and dichloromethane (1040g) were added into the reaction flask, the temperature was lowered to 5°C under nitrogen protection, and trifluoroacetic anhydride (220.5g, 1.05mol) / dichloromethane (150g) solution, keep the temperature at 5-15°C throughout the whole process, after dropping, react at room temperature for 3 hours, monitor the reaction with TLC (DCM:MeOH=25:1) until the reaction is complete; Pour into ice water (560g), stir for 20 minutes, let stand to separate the layers, separate the water phase, wash the organic phase with saturated aqueous sodium bicarbonate (100g); wash with 1M hydrochloric acid (110g), and then wash with saturated brine (200g) Washed, dried over magnesium sulfate (40 g), filtered and concentrated to obtain compound II (250.1 g), yield: 95.2%.
[0066] 2. Preparation of Compound III
[0067] Chloroacetyl chloride (101.7g, 0.9mol), nitrobenzene (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com