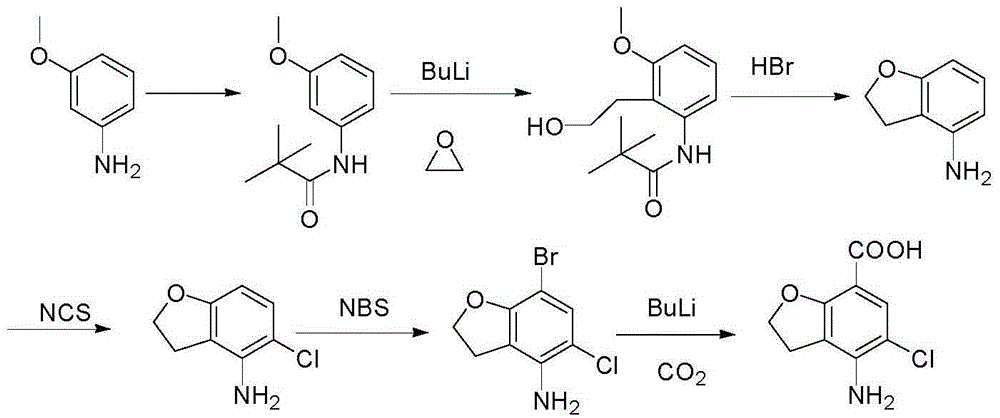
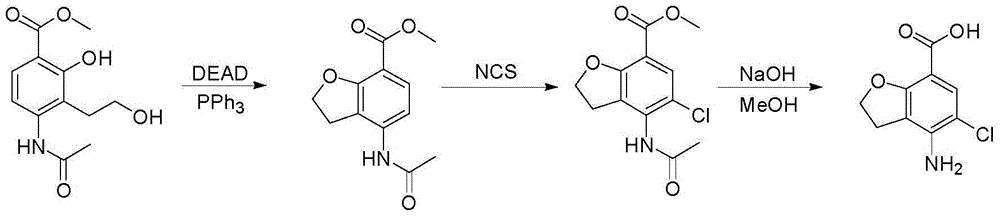
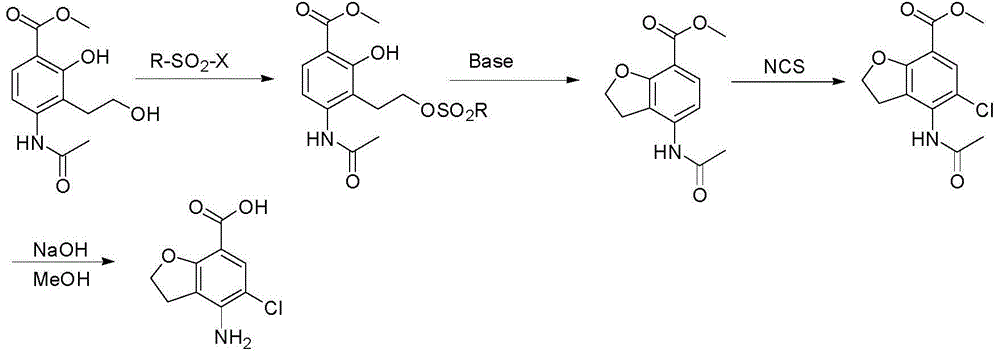
Method for synthesizing 4-amino-5-chloro-2,3-dihydro benzofuran-7-carboxylic acid
A synthesis method and technology of dihydrobenzene, applied in the field of medicine, can solve the problems of high corrosiveness and odor of pyridine, and achieve the effects of easy industrial production operation, high total yield, and easy industrial scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Add 58.6g (0.23mol) 4-(acetylamino)-2-hydroxyl-3-(2-hydroxyethyl) methyl benzoate and 200ml tetrahydrofuran respectively in a 500ml four-necked flask, 61g (0.23mol ) triphenylphosphine, stirred, added dropwise 40g (0.23mol) diethyl azodicarboxylate, continued to stir for 2 hours, added 90ml n-heptane, stirred for 3 hours, precipitated solid, and suction filtered to obtain 85g of 4-acetyl The crude product of methyl amino-2,3-dihydrobenzofuran-7-carboxylate was directly used in the next step;
[0040] 2) Add 85g (0.36mol) of the crude 4-acetamido-2,3-dihydrobenzofuran-7-carboxylate crude product and 90ml DMF into a 500ml four-neck flask, dissolve the solid, add 22g (0.13mol, 0.36 eq) N-chlorosuccinimide, stir, heat the reaction solution with an oil bath to 80-85°C, continue the reaction for 4 hours, lower the temperature of the reaction solution to room temperature, add 300ml of water, stir for 10 minutes, and precipitate a large amount of light yellow solid , suctio...
Embodiment 2
[0043] 1) Add 5.86g (0.023mol) 4-(acetylamino)-2-hydroxyl-3-(2-hydroxyethyl) methyl benzoate and 30ml tetrahydrofuran to a 100ml four-neck flask, 3.0g (0.012 mol, 0.5eq) triphenylphosphine, stirred, added dropwise 2.0g (0.012mol, 0.5eq) diethyl azodicarboxylate, continued to stir for 2 hours, added 10ml of n-hexane, stirred for 3 hours, precipitated solid, suction filtered , to obtain 6.3 g of crude 4-acetylamino-2,3-dihydrobenzofuran-7-carboxylic acid methyl ester, which was directly used in the next step;
[0044] 2) Add 85g (0.36mol) of the crude 4-acetamido-2,3-dihydrobenzofuran-7-carboxylate crude product and 90ml of acetonitrile into a 500ml four-neck flask, dissolve the solid, add 63g (0.13mol, 1eq ) N-chlorosuccinimide, stirred, heated the reaction solution with an oil bath to 60-70°C, continued the reaction for 4 hours, lowered the temperature of the reaction solution to room temperature, added 300ml of water, stirred for 10 minutes, and precipitated a large amount of...
Embodiment 3
[0047] 1) Add 5.86g (0.023mol) 4-(acetylamino)-2-hydroxyl-3-(2-hydroxyethyl) methyl benzoate and 30ml tetrahydrofuran to a 100ml four-necked flask, 9.15g (0.035mol) mol, 1.5eq) triphenylphosphine, stirred, added dropwise 6g (0.092mol, 1.5eq) diethyl azodicarboxylate, continued to stir for 2 hours, added 10ml of n-pentane, stirred for 3 hours, precipitated solid, suction filtered , to obtain 12g crude product of methyl 4-acetylamino-2,3-dihydrobenzofuran-7-carboxylate, which was directly used in the next step;
[0048] 2) Add 85g (0.36mol) of the crude 4-acetamido-2,3-dihydrobenzofuran-7-carboxylate crude product and 90ml toluene to a 500ml four-neck flask, dissolve the solid, add 63g (0.13mol, 1eq ) N-chlorosuccinimide, stirring, heating the reaction solution with an oil bath to 90-100°C, continuing the reaction for 6 hours, lowering the temperature of the reaction solution to room temperature, stirring for 10 minutes, a large amount of light yellow solid precipitated, suction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com