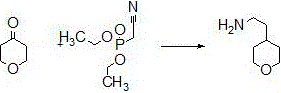
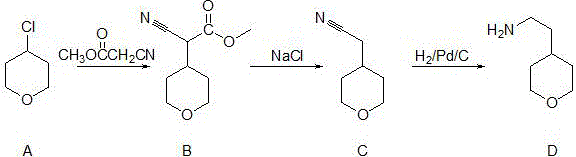
Synthetic method of 4-(2-aminoethyl) tetrahydropyrane
A tetrahydropyran and synthesis method technology, applied in the field of synthesis of chemical drug intermediates, can solve the problems of unsuitability for industrial production, instability of sodium azide, and easy explosion, and achieve low price, simple post-treatment, and reaction The effect of short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of 4-(2-aminoethyl)tetrahydropyran
[0032] The molar ratio of 4-chlorotetrahydropyran to methyl cyanoacetate is 0.9:1; the hydrogen pressure is 1MPa
[0033] (1) 80.4g (0.67mol) of 4-chlorotetrahydropyran A, 73g (0.74mol) of methyl cyanoacetate, 75g (0.67mol) of potassium tert-butoxide were dissolved in 100ml of toluene, stirred and reacted at 50°C for 12h Afterwards, the temperature was raised to 110°C for 12 hours. After the reaction was completed, the reaction solution was washed 3 times with 300ml of water, and the organic phase was concentrated under reduced pressure to obtain the oily product 2-cyano-2-(tetrahydro-4-pyranyl)acetic acid methyl ester 113.6g (0.62mol);
[0034] (2) Synthesis of (tetrahydro-4-pyranyl)-acetonitrile
[0035] Add 113.6g (0.62mol) of methyl 2-cyano-2-(tetrahydro-4-pyranyl)acetate, 10.8ml of water, 10.8g of sodium chloride, and 540ml of dimethyl sulfoxide into the flask, at 150°C Stir and react for 8 hours, distill,...
Embodiment 2
[0040] Synthesis of 4-(2-Aminoethyl)tetrahydropyran
[0041] The molar ratio of 4-chlorotetrahydropyran and methyl cyanoacetate is 1:1; the hydrogen pressure is 1MPa
[0042](1) 80.4g (0.67mol) of 4-chlorotetrahydropyran A, 66.3g (0.67mol) of methyl cyanoacetate, 75g (0.67mol) of potassium tert-butoxide were dissolved in 100ml of toluene, and the reaction was stirred at 50°C After 12 hours, the temperature was raised to 110°C and reacted for 12 hours. After the reaction was completed, the reaction solution was washed with 300ml water for 3 times, and the organic phase was concentrated under reduced pressure to obtain oily 2-cyano-2-(tetrahydro-4-pyranyl)methyl acetate Esters 110.6g (0.60mol);
[0043] (2) Synthesis of (tetrahydro-4-pyranyl)-acetonitrile
[0044] Add 110.6g (0.60mol) of methyl 2-cyano-2-(tetrahydro-4-pyranyl)acetate, 10.8ml of water, 10.8g of sodium chloride, and 540ml of dimethyl sulfoxide into the flask, at 150°C Stir and react for 8 hours, distill, add 10...
Embodiment 3
[0049] Synthesis of 4-(2-Aminoethyl)tetrahydropyran
[0050] The molar ratio of 4-chlorotetrahydropyran to methyl cyanoacetate is 0.9:1; the hydrogen pressure is 2MPa
[0051] (1) 80.4g (0.67mol) of 4-chlorotetrahydropyran A, 73g (0.74mol) of methyl cyanoacetate, 75g (0.67mol) of potassium tert-butoxide were dissolved in 100ml of toluene, stirred and reacted at 50°C for 12h Afterwards, the temperature was raised to 110°C for 12 hours. After the reaction was completed, the reaction solution was washed 3 times with 300ml of water, and the organic phase was concentrated under reduced pressure to obtain the oily product 2-cyano-2-(tetrahydro-4-pyranyl)acetic acid methyl ester 112.4g (0.61mol);
[0052] (2) Synthesis of (tetrahydro-4-pyranyl)-acetonitrile
[0053] Add 112.4g (0.61mol) of methyl 2-cyano-2-(tetrahydro-4-pyranyl)acetate, 10.8ml of water, 10.8g of sodium chloride, and 540ml of dimethyl sulfoxide into the flask, at 150°C Stir and react for 8 hours, distill, add 1000m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com