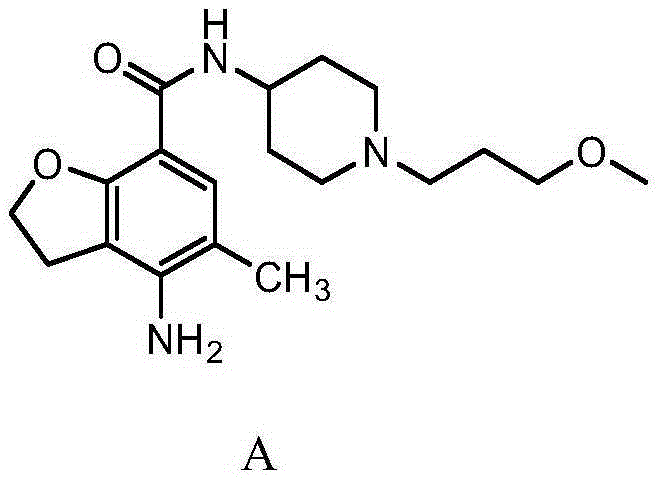
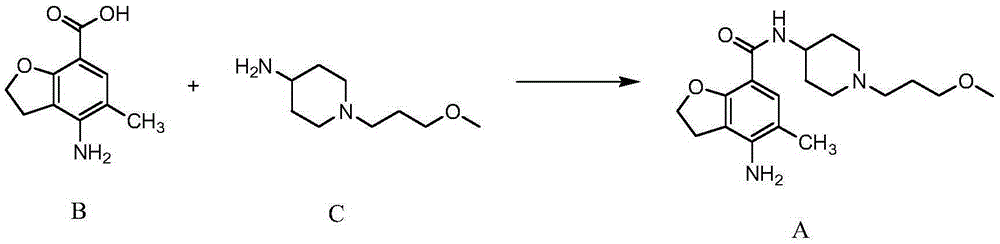
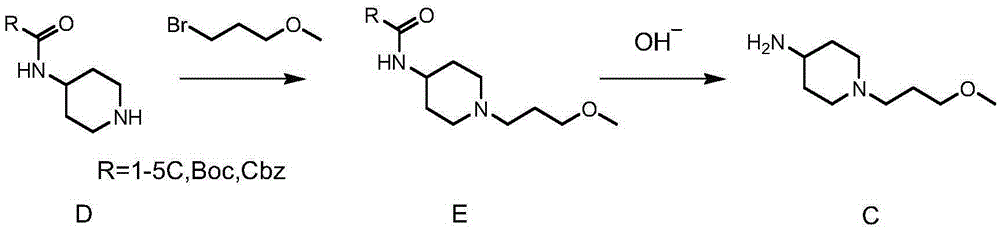
A kind of synthetic method of n-(3-methoxypropyl)-4-aminopiperidine
A technology of methoxypropyl and aminopiperidine dihydrochloride, which is applied in the field of preparation of pharmaceutical intermediate compounds, can solve problems such as difficult filtration and difficult industrialization, and achieve easy handling and storage, simple operation and low price cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0060]
[0061] At room temperature, in a 250ml three-necked flask, add 17.1g N-(3-methoxypropyl)piperidone, 10.7g benzylamine, 100ml methanol, stir and dissolve, then add 21.2g triacetoxyhydroboration After adding sodium, after stirring at room temperature for 4 hours, the conversion was complete as monitored by TLC. Add 100ml of dilute hydrochloric acid with a concentration of 0.1mol / L to quench, evaporate the solvent under reduced pressure, add 100ml of water and 100ml of ethyl acetate, stir and separate, keep the organic layer, extract the aqueous layer twice with ethyl acetate, and combine the organic Mutually. The organic phase was washed twice with water, dried over anhydrous magnesium sulfate for 4 hours and concentrated to obtain 21.6 g of an oily product with a molar yield of 82.3%.
[0062] Example 2-5 is to screen the substituted benzylamine of this step reaction, and the charging ratio and experimental operation are all the same as Example 1, and the results a...
example 4
[0065] The concrete operation of example 4 is as follows:
[0066] At room temperature, in a 250ml three-necked flask, add 17.1g of N-(3-methoxypropyl)piperidone, 13.7g of p-methoxybenzylamine, and 100ml of methanol. After stirring and dissolving, add 21.2g of three After sodium acetoxyborohydride was stirred at room temperature for 4 hours, the conversion was complete as monitored by TLC. Add 100ml of dilute hydrochloric acid with a concentration of 0.1mol / L to quench, evaporate the solvent under reduced pressure, add 100ml of water and 100ml of ethyl acetate, stir and separate, keep the organic layer, extract the aqueous layer twice with ethyl acetate, and combine The organic phase. The organic phase was washed twice with water, dried over anhydrous magnesium sulfate for 4 hours and concentrated to obtain 23.2 g of an oily product with a molar yield of 88.6%.
[0067] Example 6-7 is to screen the solvent of this step reaction, and charging ratio and operation are identical...
example 11
[0072]
[0073] At room temperature, in a 250ml three-neck flask, add 13.1g of intermediate C-1 and 100ml of methanol, stir until dissolved, add 3g of 10% palladium carbon, pass in hydrogen under normal pressure, and react for more than 12 hours. After the conversion of raw materials is monitored by TLC , filter out the palladium carbon, add dropwise 100ml concentration of 2mol / L HCl ethanol solution to the filtrate, after a large amount of solids appear, continue to stir for more than 4 hours, filter to obtain a white solid, weigh 11.8g after drying, and the molar yield is 96.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com