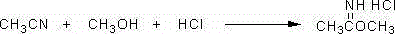Preparation process for cyanomethyl ester
A cyanomethyl ester and preparation technology, which is applied in the field of cyanomethyl ester preparation technology, can solve the problems of product yield reduction, hydrochloride is prone to side reactions, etc., and achieve the effect of smooth reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Add 287Kg (7Kmol) of acetonitrile, 224Kg (7Kmol) of methanol, and 520Kg of solvent toluene to a 3000L synthesis reactor, start stirring and freezing brine, and cool the materials in the reactor to 0-5°C. At the above temperature, start to feed 268.5Kg (7.36Kmol) of hydrogen chloride gas, usually about 10 hours. After the ventilation is over, the material in the reactor is gradually raised to 25-30°C, and the salt-forming reaction is started for 7 hours, and the salt-forming reaction is completed.
[0024] Under stirring, cool the reaction solution to 0°C, slowly add 50Kg of ammonium carbonate, and then add 1050Kg (7Kmol) of 28% cyanamide aqueous solution, and adjust the pH value to 6.0-6.5 with 30% aqueous sodium hydroxide solution. -20 ℃ heat preservation reaction for 1 hour, the ester-forming reaction is completed.
[0025] After standing still for half an hour, the organic layer was separated and recovered, and the organic layer was vacuum distilled to ob...
Embodiment 2
[0026] Example 2: Add 287Kg (7Kmol) of acetonitrile, 224Kg (7Kmol) of methanol, and 574Kg of solvent toluene to a 3000L synthesis reactor, start stirring and freezing brine, and cool the materials in the reactor to 0-5°C. At the above temperature, start to feed 270Kg (7.40Kmol) of hydrogen chloride gas, usually about 9 hours. After the aeration, the material in the reactor is gradually raised to 25-30°C, and the salt-forming reaction is started for 6 hours, and the salt-forming reaction is completed.
[0027] Under stirring, cool the reaction solution to 0°C, slowly add 50Kg of ammonium carbonate, and then add 1050Kg (7Kmol) of 28% cyanamide aqueous solution, and adjust the pH value to 6.0-6.5 with 30% aqueous sodium hydroxide solution. -20 ℃ heat preservation reaction for 1 hour, the ester-forming reaction is completed.
[0028] After standing still for half an hour, the organic layer was separated and recovered, and the organic layer was vacuum distilled to obtain the produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com