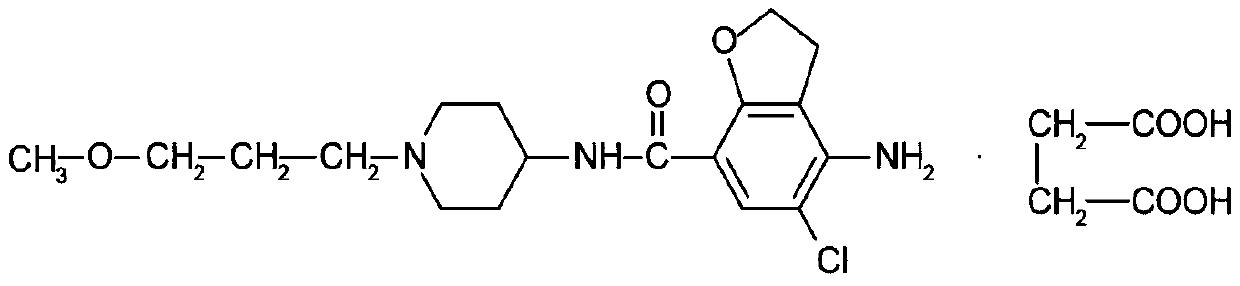
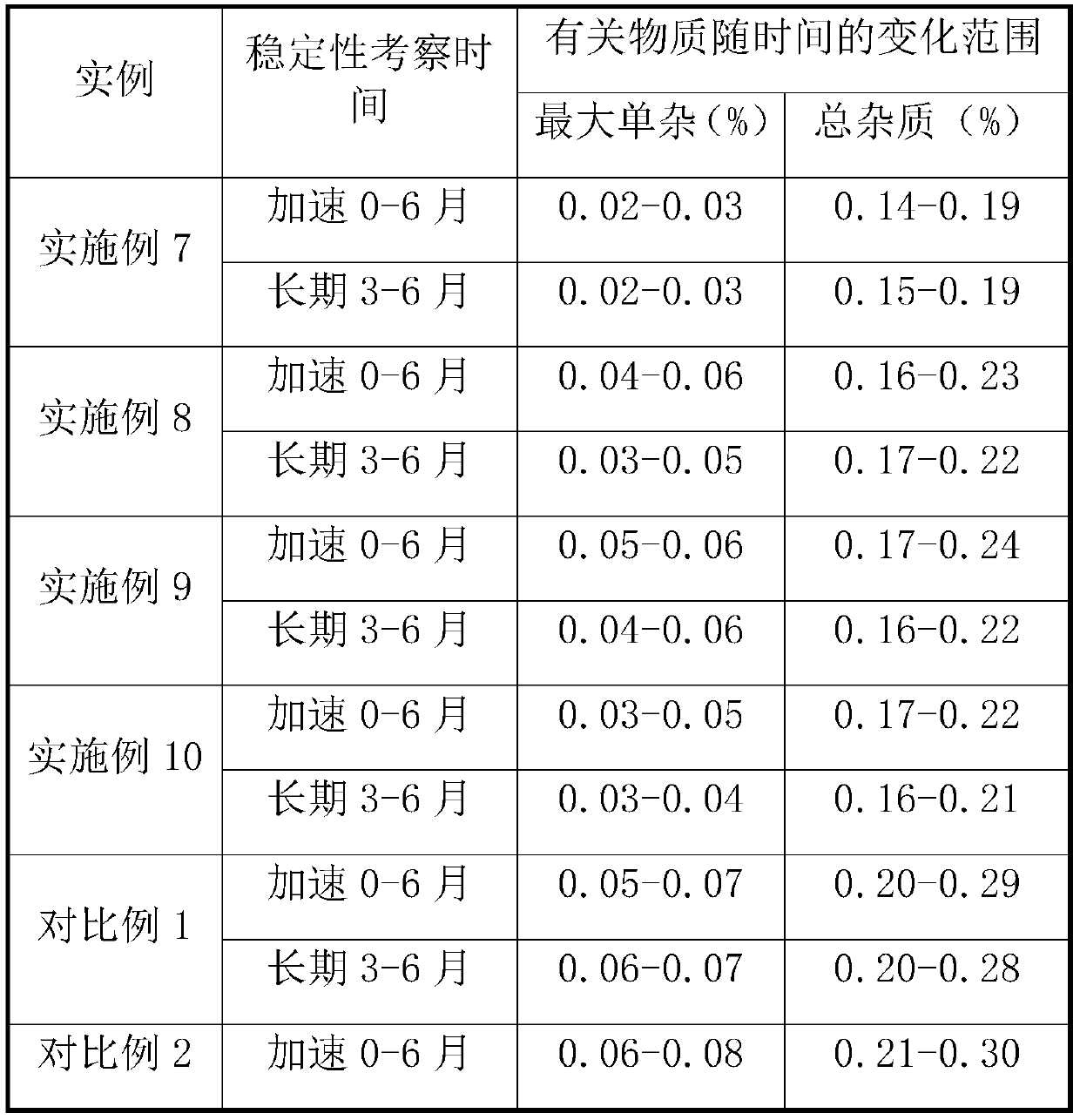
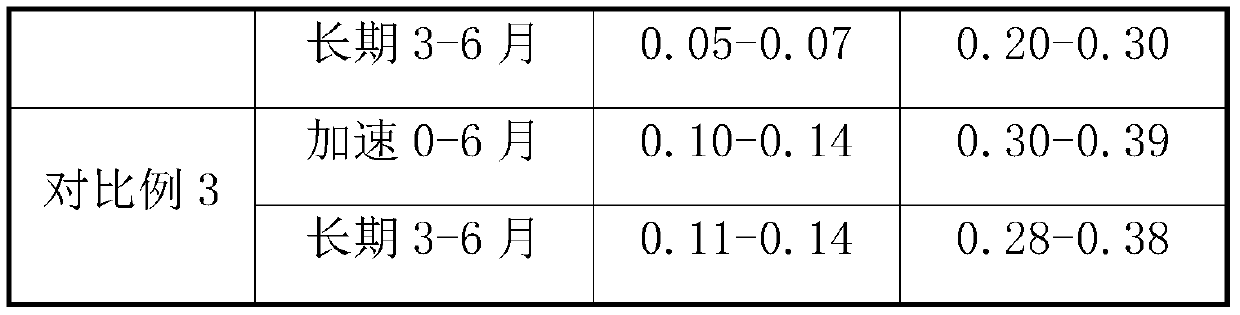
A kind of prucalopride succinate tablet and preparation method thereof
A technology of prucalopride and succinic acid, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low stability and prucalopride succinate tablets Weak stability and other problems, to achieve the effect of good stability, flexible medication time, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 A kind of prucalopride succinate tablet tablet and preparation method thereof
[0049] Formula: by weight: 1 part of prucalopride succinate (calculated as prucalopride), 70 parts of lactose, 10 parts of microcrystalline cellulose, 10 parts of sodium carboxymethyl starch, 1 part of silicon dioxide, hard 0.2 parts of magnesium fatty acid, 3 parts of Opadry, 12 parts of laminin, and 24 parts of trehalose. Preparation:
[0050] (1) Pulverize the prucalopride succinate in the formula dosage through an 80-mesh sieve to obtain powder A; pass the lactose in the formula dosage through a 80-mesh sieve to obtain powder B;
[0051] (2) Place the microcrystalline cellulose in the formula dosage, powder A and powder B obtained in step (1) in a wet mixing granulator, adjust the stirring speed to 150r / min, and mix for 18min to obtain material A;
[0052] (3) Sodium carboxymethyl starch, laminin, trehalose and the mixed material A obtained in step (3) are placed in the bl...
Embodiment 2
[0055] Embodiment 2 A kind of prucalopride succinate tablet and preparation method thereof
[0056] Formula: by weight: 1 part of prucalopride succinate (calculated as prucalopride), 100 parts of mannitol, 15 parts of povidone, 15 parts of sodium carboxymethyl starch, 1.1 parts of silicon dioxide, talc 0.55 parts of powder, 4 parts of Opadry, 15 parts of laminin, and 25 parts of trehalose.
[0057] Preparation method: The difference from Example 1 is that the raw materials are different, the air inlet temperature in step (5) is 55° C., and the material temperature is 40° C.; the rest are the same as in Example 1.
Embodiment 3
[0058] Embodiment 3 a kind of prucalopride succinate tablet and preparation method thereof
[0059] Formula: by weight: 1 part of prucalopride succinate (calculated as prucalopride), 110 parts of lactose, 18 parts of sucrose, 20 parts of sodium carboxymethyl starch, 1.2 parts of silicon dioxide, 0.6 parts of micronized silica gel , 5 parts of Opadry, 18 parts of laminacin, 26 parts of trehalose.
[0060] Preparation method: The difference from Example 1 is that the raw materials are different, the inlet air temperature in step (5) is .60°C, and the material temperature is 45°C; the rest are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com