Prucalopride succinate tablet composition and preparation method thereof
A technology of prucalopride and succinic acid, which is applied in the direction of drug combinations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of time-consuming, labor-intensive, and complex equipment that are not suitable for industrial production and other problems, achieve high uniformity and bioavailability, solve the problem of content uniformity, and improve the effect of content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A composition of prucalopride succinate, calculated as prucalopride, comprises the following ingredients:
[0040]
[0041]
[0042] The average particle size of prucalopride succinate was 5.551 microns with a particle size distribution of D90<10 microns.
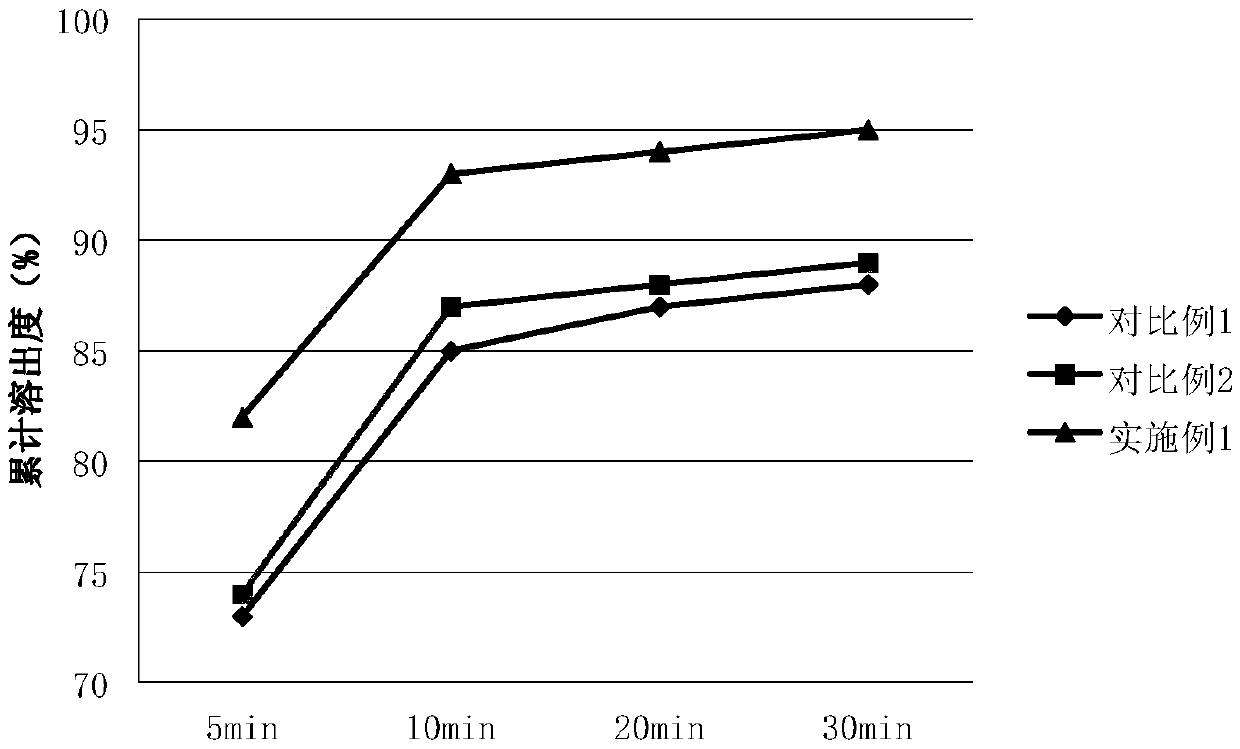
[0043] In order to confirm the impact of the average particle size of the bulk drug on the composition, only the average particle size of the bulk drug prucalopride succinate was changed when other conditions were the same, and compared with comparative examples 1 and 2 with larger average particle sizes. The specific comparison results are shown in Table 1 below:
[0044] Table 1 Content uniformity and dissolution rate data of prucalopride succinate with different particle sizes
[0045]
[0046] Provided in Table 1 within the scope of the composition of the present invention, the dissolution and content uniformity of the formulation obtained by changing the average particle size of prucalopride succinate ...
Embodiment 2
[0048] A composition of prucalopride succinate, calculated as prucalopride, comprises the following components:
[0049]
[0050] The average particle size of prucalopride succinate was 6.395 microns with a particle size distribution of D90<10 microns.
Embodiment 3
[0052] A composition of prucalopride succinate, calculated as prucalopride, comprises the following components:
[0053]
[0054] The average particle size of prucalopride succinate was 6.395 microns with a particle size distribution of D90<10 microns.
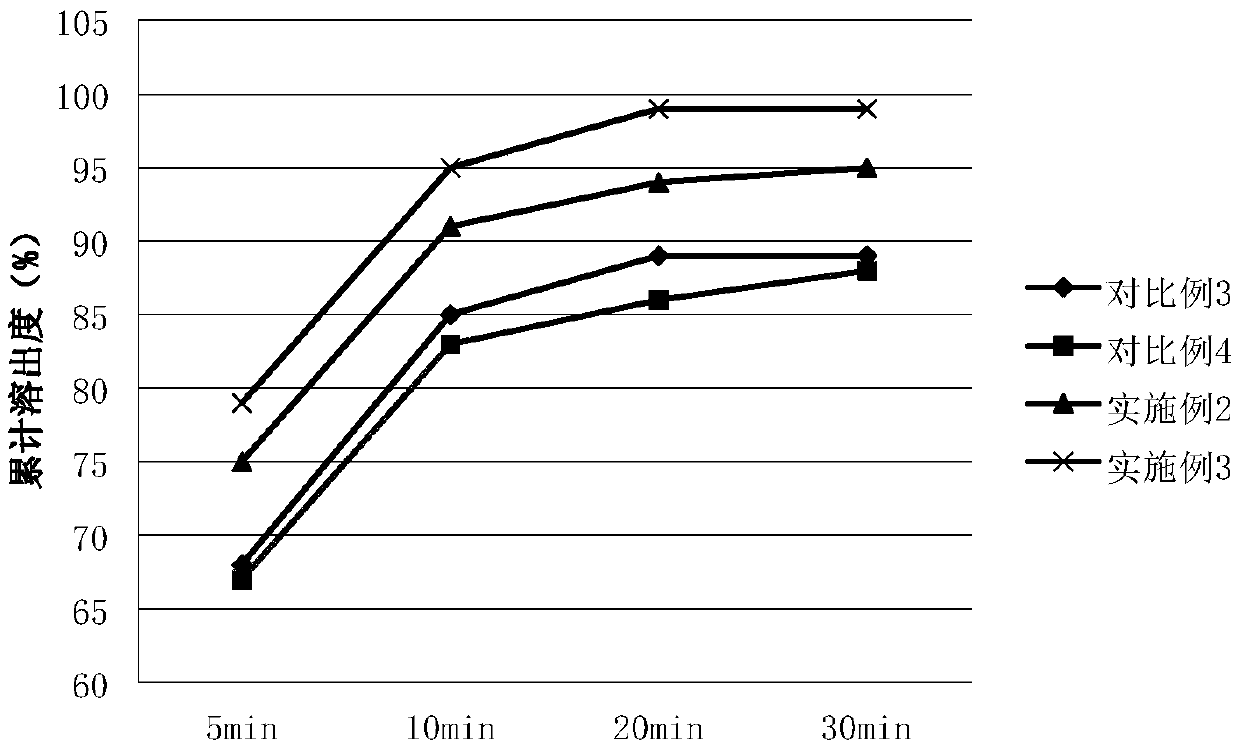
[0055] In order to confirm the impact of the amount of silicon dioxide added on the composition, only the added amount of silicon dioxide was changed when other conditions were the same, and the examples 2 and 3 with more silicon dioxide added were compared with the silicon dioxide added. Comparative examples 3 and 4 are compared, and the specific comparison results are shown in Table 2 below:
[0056] Table 2 Content uniformity and dissolution data at different additions of silica
[0057]
[0058]
[0059] Table 2 shows the angle of repose and the dissolution rate comparison data of not adding silicon dioxide, adding a small amount of silicon dioxide and adding the required amount of silicon dioxide in the present ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com