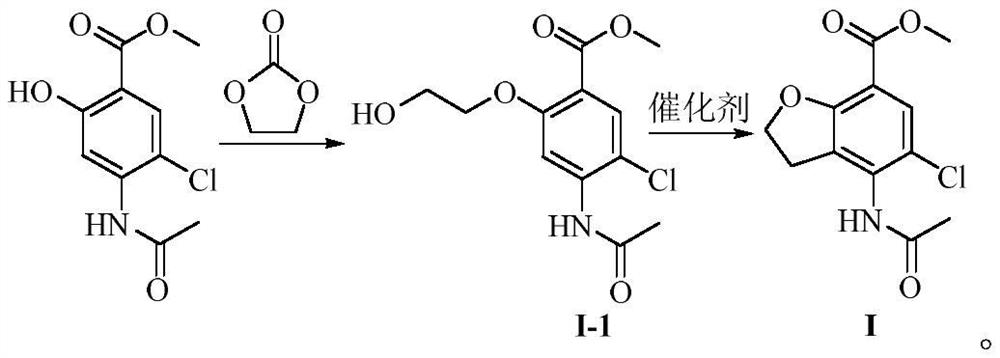
Preparation method of 4-acetamido-5-chloro-2, 3-dihydrobenzofuran-7-carboxylic acid methyl ester
A technology of acetamido and methyl carboxylate, applied in organic chemistry, bulk chemical production, etc., to achieve the effects of high yield and purity, shortened reaction route, and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Methyl 4-acetamido-5-chlorosalicylate (methyl 4-acetamido-2-hydroxy-5-chlorobenzoate, 24.36 g, 0.1 mol), potassium carbonate (17.97 g, 0.13 mol) were added In N,N-dimethylformamide (250ml), add ethylene carbonate (15.85g, 0.18mol) under temperature control at 10~15℃, and after the addition, control temperature at 100~105℃ to react. The reaction solution was cooled to room temperature for filtration, dichloromethane (150ml) was added to the filtrate, the organic phase was separated and the organic phase was washed with saturated brine (150ml×2), dried, filtered, and the filtrate was concentrated under reduced pressure to dryness to be the intermediate I-1, yield 93.7%.
Embodiment 2
[0067] Methyl 4-acetamido-5-chlorosalicylate (methyl 4-acetamido-2-hydroxy-5-chlorobenzoate, 24.34g, 0.1mol), sodium bicarbonate (10.92g, 0.13mol) Add N,N-dimethylacetamide (250ml), control the temperature at 10~15℃, add ethylene carbonate (11.45g, 0.13mol), and after the addition, control the temperature at 100~105℃ to react, after testing the reaction is completed , the reaction solution was cooled to room temperature and filtered, dichloromethane (150ml) was added to the filtrate, the organic phase was separated and the organic phase was washed with saturated brine (150ml×2), dried, filtered, and the filtrate was concentrated under reduced pressure to dryness. Body I-1, yield 90.6%.
Embodiment 3
[0069] Methyl 4-acetamido-5-chlorosalicylate (methyl 4-acetamido-2-hydroxy-5-chlorobenzoate, 24.38g, 0.1mol), triethylamine (13.16g, 0.13mol) Add dimethyl sulfoxide (250ml), add ethylene carbonate (10.57g, 0.12mol) under temperature control at 10-15°C, and complete the addition, control temperature at 110-115°C for reaction, and after the reaction is detected, reduce the reaction solution to a lower temperature. Filter to room temperature, add dichloromethane (150ml) to the filtrate, separate the organic phase, wash the organic phase with saturated brine (150ml×2), dry, filter, and concentrate the filtrate to dryness under reduced pressure to obtain Intermediate I-1, Yield 87.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com