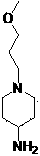Preparation method of Prucalopride intermediates
An intermediate, methoxypropyl technology, applied in the field of medicinal chemistry
Inactive Publication Date: 2013-10-16
BEIJING VENTUREPHARM BIOTECH
View PDF1 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
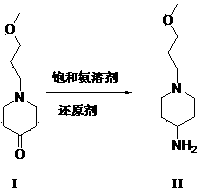
This article mainly involves the synthesis of 1-(3-methoxypropyl)-4-piperidinamine, an important intermediate of prucalopride. 3-methoxypropyl)-4-piperidone can be used as a raw material to prepare compound I by high-pressure hydrogenation, but this method uses palladium carbon and is expensive, and industrial hydrogenation has safety concerns. Improved, the improved method is easy to operate, the reaction conditions are mild, the yield is high, and it is suitable for large-scale industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0012] Example 2:
Embodiment 2
[0014] Example 3:
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention belongs to the field of medicine chemistry, and specifically relates to a preparation method of Prucalopride intermediates 1-(3-Methoxypropyl)-4-piperidinamine. The preparation method comprises following steps: dissolving 1-(3-Methoxypropyl)-4-piperidone (I) in an organic solvent, adding in saturated ammonia solution or ammonia salt and a reducing agent so as to obtain 1-(3-Methoxypropyl)-4-piperidinamine (II). The preparation method of Prucalopride intermediates has the advantages of simple operation, mild reaction conditions, high yield, and suitability for mass industry production.
Description
technical field [0001] The invention belongs to the field of medicinal chemistry and relates to a method for preparing 1-(3-methoxypropyl)-4-piperidinamine, an important intermediate of prucalopride (see I for the structure). [0002] [0003] I Background technique [0004] In recent years, with the impact of many factors such as living standards, lifestyles, and dietary structure, coupled with the rapid increase of the elderly population, the incidence of constipation in my country is on the rise. The huge consumer group of patients will strongly stimulate the continuous expansion of the constipation treatment drug market. [0005] Prucalopride is a selective 5-HT developed by Movetis NV in Belgium--------------------------------- ------------- 4 Receptor agonist, in October 2009, the European Medicines Agency approved the marketing of its monosuccinate, which is clinically used to treat female constipation that cannot be relieved by laxatives. For patients with va...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D211/58
Inventor 侯艳超闫起强马苏峰王进敏
Owner BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com