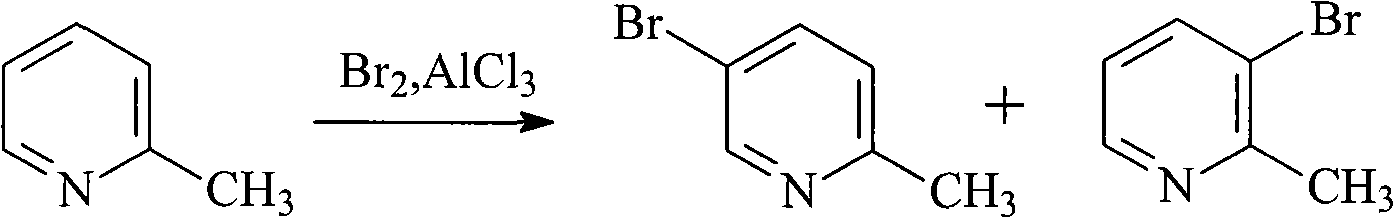
Method for preparing 5-bromo-2-methylpyridine
A technology of picoline and chloropyridine, which is applied in the field of preparation of intermediate 5-bromo-2-picoline, can solve the problems of large amount of aluminum trichloride, difficulty in industrialized production, low product yield and the like, and can achieve production Low cost, good catalytic effect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
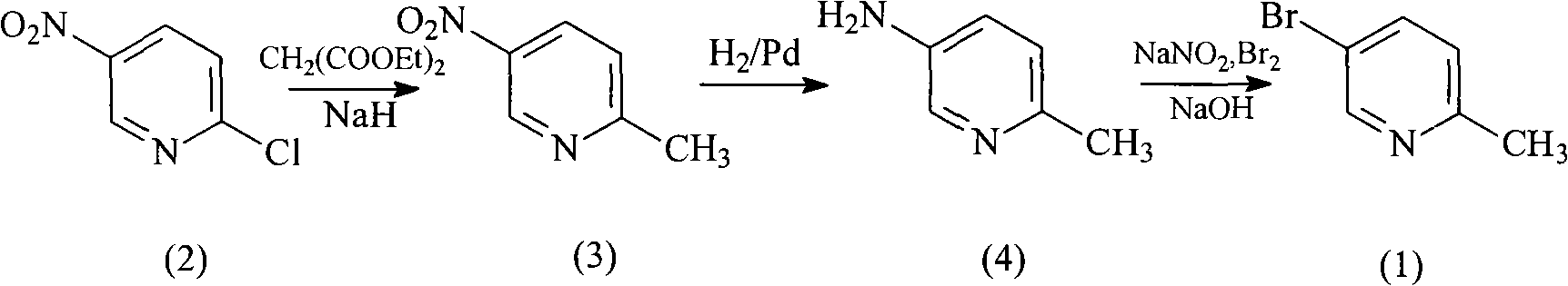
[0017] Embodiment 1: the preparation of 5-nitro-2-picoline (3)
[0018] A mixture of diethyl malonate (42 mL, 1.28 mol) and sodium hydrogen (4.8 g, 0.2 mol) was slowly heated to 90°C, stirred for 1 h, then heated to 120°C and stirred for 30 min, then cooled to room temperature. A solution of 5-nitro-2-chloropyridine (2) (25 g, 0.16 mol) in toluene (200 mL) was added dropwise. After the addition was complete, the reaction solution was heated to 110° C. for 1.5 h, cooled to room temperature and stirred for 15 h. The solvent was evaporated under reduced pressure, 6N HCl (200 mL) was added, the temperature was raised to reflux for 4 h and then cooled to room temperature. Make alkaline with sodium carbonate, extract with ethyl acetate (6×100 mL), combine organic phases, and dry over anhydrous sodium sulfate for 6 h. After suction filtration, the filtrate was evaporated to dryness under reduced pressure to obtain the crude product 5-nitro-2-picoline (3), 20.4 g, 94%. 1 HNMR (400MH...
Embodiment 2
[0019] Embodiment 2: Preparation of 5-nitro-2-picoline (3)
[0020] A mixture of diethyl malonate (42 mL, 1.28 mol) and sodium (4.6 g, 0.2 mol) was slowly warmed up to 90°C, stirred for 2 h, then raised to 100°C and stirred for 50 min, then cooled to room temperature. A solution of 5-nitro-2-chloropyridine (2) (25 g, 0.16 mol) in toluene (200 mL) was added dropwise. After the addition was complete, the reaction solution was heated to 110° C. for 1.5 h, cooled to room temperature and stirred for 15 h. The solvent was evaporated under reduced pressure, 6N HCl (200 mL) was added, the temperature was raised to reflux for 4 h and then cooled to room temperature. Make alkaline with sodium carbonate, extract with ethyl acetate (6×100 mL), combine organic phases, and dry over anhydrous sodium sulfate for 6 h. After suction filtration, the filtrate was evaporated to dryness under reduced pressure to obtain the crude product 5-nitro-2-picoline (3), 20.0 g, 93%. 1 HNMR (400MHz, CDCl 3...
Embodiment 3
[0021] Embodiment 3: Preparation of 5-amino-2-picoline (4)
[0022] 5-Nitro-2-methylpyridine (3) (13g, 94.1mmoL) and 10%Pd / C(0.1)1,4-dioxane solution was passed into hydrogen until the pressure was 0.4MPa, and the temperature was raised to 30 °C, reacted for 16h. Cool to room temperature, filter, and evaporate the filtrate to dryness under reduced pressure to give 5-amino-2-picoline (4) as a solid, 9.9 g, 97%. 1 HNMR (400MHz, CDCl 3 ): δ8.61(s, 1H), 7.29(m, 1H), 7.25(m, 1H), 4.0(s, 2H), 2.55(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com