Synthetic method of p-nitroanisole
A technology of p-nitroanisole and synthetic method, which is applied in the field of synthesis of p-nitroanisole, can solve the problems of low yield of nitroanisole, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
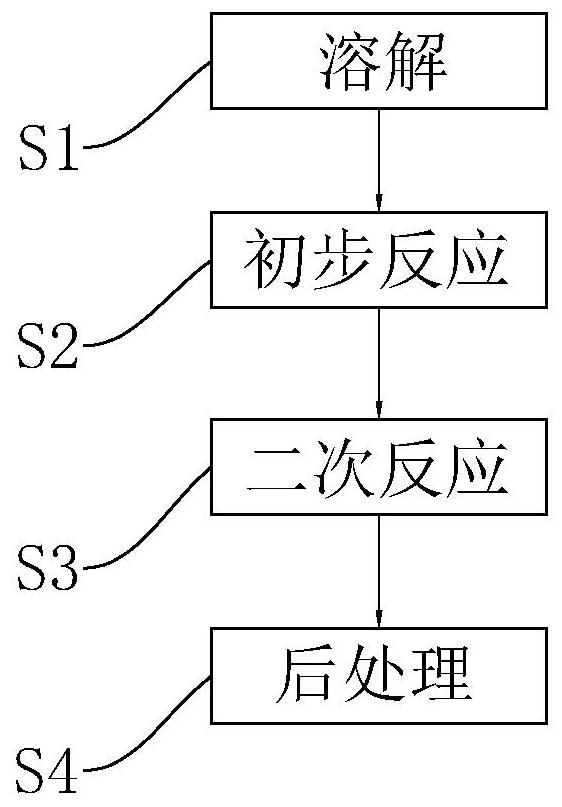
[0039] refer to figure 1 , the present embodiment discloses a synthetic method of p-nitroanisole, which comprises the following steps:
[0040] S1: dissolved. In a 500mL three-neck flask, first add 70mL of organic solvent, the organic solvent is ethanol; then add reactant methanol 2.4mol. Then 0.48 mol of sodium hydroxide powder was added. Then use a mechanical stirrer to continuously stir; and install a condenser to condense and reflux. After observing that the sodium hydroxide is completely dissolved, the first mixed solution is formed.
[0041] S2: Initial response. Add 0.3 mol of p-nitrochlorobenzene into the three-necked flask where the first mixed solution is located, keep the reaction conditions and environment in step S1 and continue the reaction for 2.5 hours to obtain the second mixed solution.
[0042] S3: Secondary reaction. 0.512g of catalyst was added into the second mixed liquid, the catalyst was tetrapropylammonium bromide, and the reaction conditions and...
Embodiment 2
[0045] S1: dissolved. In a 500mL three-neck flask, first add 70mL of organic solvent, the organic solvent is ethanol; then add reactant methanol 2.4mol. Then 0.48 mol of sodium hydroxide powder was added. Then use a mechanical stirrer to continuously stir; and install a condenser to condense and reflux. After observing that the sodium hydroxide is completely dissolved, the first mixed solution is formed.
[0046] S2: Initial response. Add 0.3 mol of p-nitrochlorobenzene into the three-necked flask where the first mixed solution is located, keep the reaction conditions and environment in step S1 and continue the reaction for 2.5 hours to obtain the second mixed solution.
[0047]S3: Secondary reaction. Add 0.512g of catalyst into the second mixed liquid, choose tetrabutylammonium bromide as the catalyst, and keep the reaction conditions and environment of S1 for 6h.
[0048] S4: post-processing. The material in the three-necked flask after S3 treatment was directly filter...
Embodiment 3
[0050] S1: dissolved. In a 500mL three-neck flask, first add 70mL of organic solvent, the organic solvent is ethanol; then add reactant methanol 2.4mol. Then 0.48 mol of sodium hydroxide powder was added. Then use a mechanical stirrer to continuously stir; and install a condenser to condense and reflux. After observing that the sodium hydroxide is completely dissolved, the first mixed solution is formed.
[0051] S2: Initial response. Add 0.3 mol of p-nitrochlorobenzene into the three-necked flask where the first mixed solution is located, keep the reaction conditions and environment in step S1 and continue the reaction for 2.5 hours to obtain the second mixed solution.
[0052] S3: Secondary reaction. 0.512g of catalyst was added into the second mixed liquid, the catalyst was benzyltriethylammonium chloride, and the reaction conditions and environment of S1 were maintained for 6 hours.
[0053] S4: post-processing. The material in the three-necked flask after S3 treatme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com