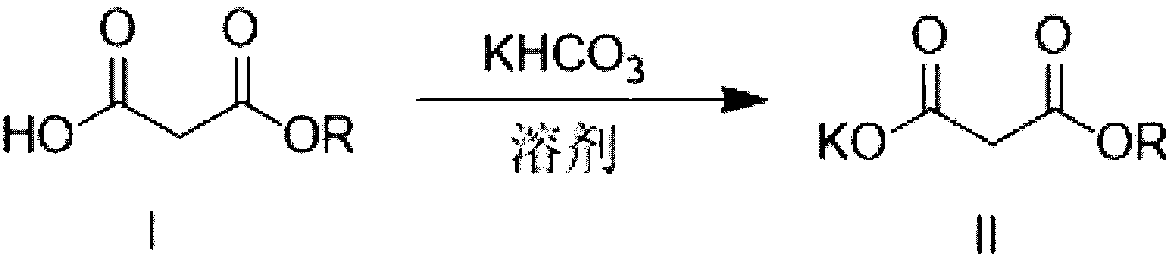
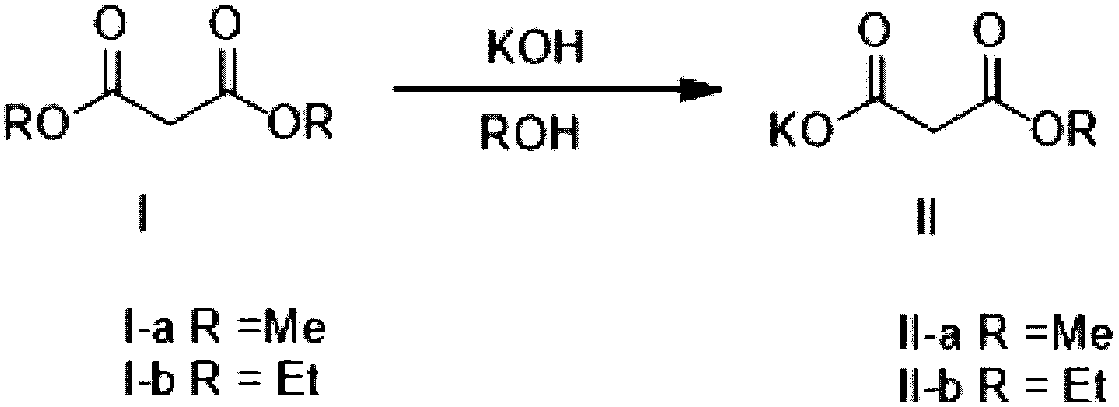
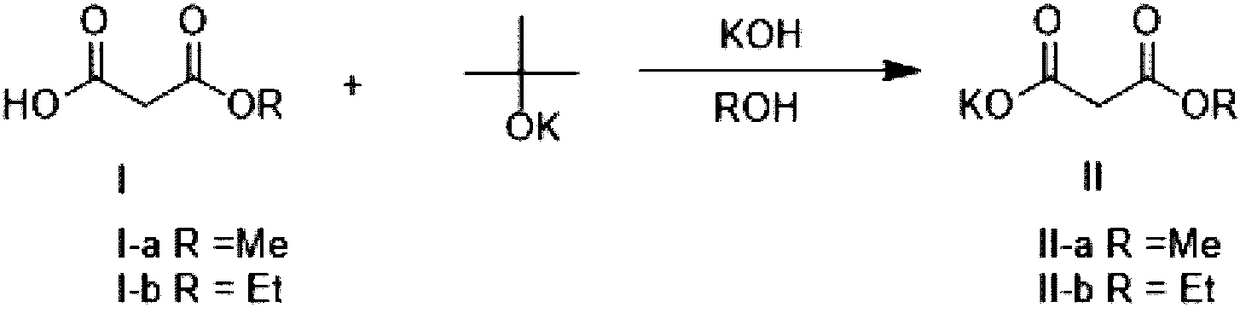
Method for preparing potassium monoethyl malonate
A technology of malonic acid monoester and potassium salt, which is applied in the preparation of carboxylic acid ester, the preparation of organic compounds, chemical instruments and methods, etc. High purity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of mono-tert-butyl malonate potassium salt
[0032] Dissolve 650 g of mono-tert-butyl malonate in 5.0 liters of dichloromethane, stir at 35-45 degrees until completely dissolved, add 398 g of solid powdered potassium bicarbonate in batches, Stir at low temperature for 30 minutes, raise the temperature to reflux, and keep the temperature for 10-15 hours to react until no gas is released and the reaction solution is clear.
[0033] Cool down, concentrate to remove most of the solvent, the product is precipitated as a white solid, filtered, and dried to obtain a white crystalline powder.
[0034] product testing:
[0035] 1 H-NMR (400MHz, D2O, 293K, TMS) δ1.42(s, 9H), 3.15(s, 2H, H-D exchange in D2O).
[0036] 13 C-NMR (100MHz, D2O, 293K, TMS) δ27.2, 46.2, 82.8, 170.8, 174.7.
Embodiment 2
[0037] Embodiment 2: the preparation of monoisopropyl malonate potassium salt
[0038] Dissolve 650g of monoisopropyl malonate in 5.0 liters of isopropanol, stir at 15-25 degrees until completely dissolved, add 405 g of solid powdered potassium bicarbonate in batches, Stir at low temperature for 30 minutes, raise the temperature to 50 degrees, and keep the temperature for 10-15 hours to react until no gas is released and the reaction solution is clear.
[0039] Cool down, concentrate to remove most of the solvent, the product is precipitated as a white solid, filtered, and dried to obtain a white crystalline powder.
[0040] product testing:
[0041] 1 H-NMR (400MHz, D2O, 293K, TMS) δ1.22(d, J=3.3Hz, 6H), 3.22(s, 2H, H-D exchange inD2O), 4.90–5.03(m, 1H).
[0042] 13 C-NMR (100MHz, D2O, 293K, TMS) δ20.9, 45.1, 70.1, 171.1, 174.3.
Embodiment 3
[0043] Embodiment 3: the preparation of monoethyl malonate potassium salt
[0044] Dissolve 300 g of monoethyl malonate in 2.5 liters of acetone, stir at 45-60 degrees until completely dissolved, add 215 g of solid powdered potassium bicarbonate in batches, and stir at 45-60 degrees for 30 Minutes, heat up to reflux, keep warm for 10-15 hours, until no gas is released, and the reaction solution is clear.
[0045] Cool down, concentrate to remove most of the solvent, the product is precipitated as a white solid, filtered, and dried to obtain a white crystalline powder.
[0046] product testing:
[0047] 1 H-NMR (400MHz, D2O, 293K, TMS) δ1.23(t, J=6.8Hz, 3H), 3.25(s, 2H, H-D exchange inD2O), 4.15(q, J=6.8Hz, 2H).
[0048] 13 C-NMR (100MHz, D2O, 293K, TMS) δ13.3, 44.8, 62.1, 171.6, 174.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com