Preparation method of triazinone ring
A technology of triazinone and cyclization reaction, which is applied in the direction of organic chemistry, can solve the problems of high process cost, complicated process, environmental protection issues, etc., and achieve the effect of reducing product loss, simple process, and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
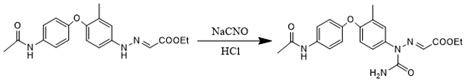
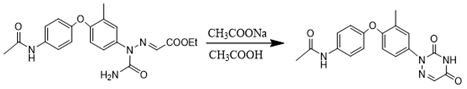
[0045] Take 10g of N-(4-(4-amino-2-methylphenoxy)phenyl)acetamide and 5.54g of potassium monoethyl malonate, add 30g of glacial acetic acid, heat up to 40°C and stir to dissolve, then mix solution, then take 2.69g of sodium nitrite in a beaker, add 6.28g of purified water to the beaker and stir to dissolve, configure it as a sodium nitrite aqueous solution with a mass fraction of 30%, control the temperature at 15°C, and slowly add the sodium nitrite aqueous solution dropwise Into the mixed solution, control the dropping time to be 1h, and then react for 1h to obtain the diazonium condensation reaction feed liquid;
[0046] Take 2.54g of sodium cyanate, add it to the diazonium condensation reaction feed solution, raise the temperature to 35°C, control the temperature at 35°C, and slowly add 6.68g of 32% hydrochloric acid dropwise, and control the dropping time for 1h. amidation liquid;
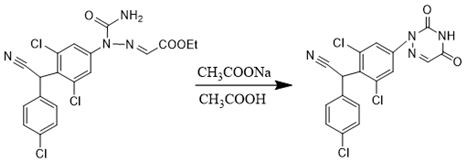
[0047] Put 4.48g of sodium acetate solid into the amidation feed solution, raise the temp...
Embodiment 2
[0049] Take 10g of N-(4-(4-amino-2-methylphenoxy)phenyl)acetamide and 5.54g of potassium monoethyl malonate, add 30g of glacial acetic acid, heat up to 40°C and stir to dissolve, then mix Solution, then take 2.69g of sodium nitrite in a beaker, add 6.28g of purified water to the beaker and stir to dissolve, configure the sodium nitrite aqueous solution with a mass fraction of 30%, control the temperature at 10°C, and slowly add the sodium nitrite aqueous solution dropwise Into the mixed solution, control the dropping time to be 1h, and then react for 1h to obtain the diazonium condensation reaction feed liquid;
[0050] Take 2.54g of sodium cyanate, add it to the diazonium condensation reaction feed solution, raise the temperature to 35°C, control the temperature at 35°C, and slowly add 6.68g of 32% hydrochloric acid dropwise, and control the dropping time for 1h. amidation liquid;
[0051] Put 4.48g of sodium acetate solid into the amidation feed solution, raise the temperat...
Embodiment 3
[0053] Take 10g of N-(4-(4-amino-2-methylphenoxy)phenyl)acetamide and 5.54g of potassium monoethyl malonate, add 30g of glacial acetic acid, heat up to 40°C and stir to dissolve, then mix solution, then take 2.69g of sodium nitrite in a beaker, add 6.28g of purified water to the beaker and stir to dissolve, configure it as a sodium nitrite aqueous solution with a mass fraction of 30%, control the temperature at 20°C, and slowly add the sodium nitrite aqueous solution dropwise Into the mixed solution, control the dropping time to be 1h, and then react for 1h to obtain the diazonium condensation reaction feed liquid;
[0054]Take 2.54g of sodium cyanate, add it to the diazonium condensation reaction feed solution, raise the temperature to 35°C, control the temperature at 35°C, and slowly add 6.68g of 32% hydrochloric acid dropwise, and control the dropping time for 1h. amidation liquid;
[0055] Put 4.48g of sodium acetate solid into the amidation feed solution, raise the tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com