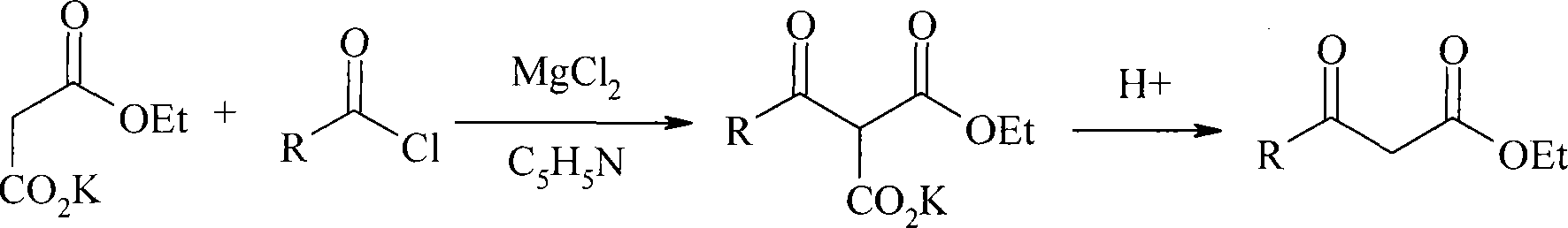
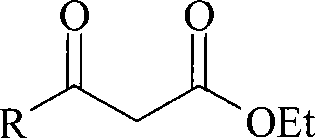
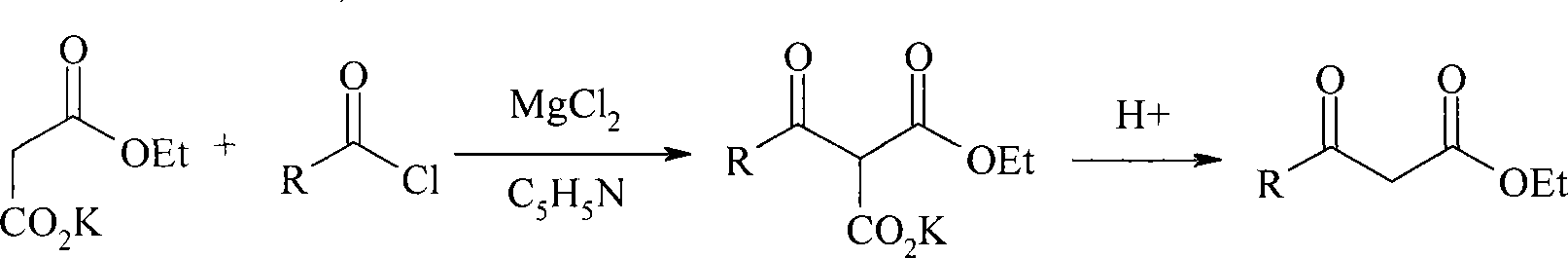
Method for preparing beta-keto acid ethyl ester
A technology of ethyl keto acid and ethyl acetate is applied in the field of preparation of ethyl keto acid, can solve the problems of unsatisfactory industrialization prospects, harsh reaction conditions, difficult post-processing and the like, and achieves stable product quality and reaction process conditions. Gentle, pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 500ml three-neck flask equipped with stirring, a thermometer and a dropping funnel, add 13.6g (0.08mol) potassium monoethyl malonate and 125ml ethyl acetate respectively, and slowly add 9.5g (0.097mol) anhydrous MgCl under stirring. 2 And 28ml (0.2mol) pyridine, react at room temperature for 5 hours. Cool, control the temperature at 0-5°C, add 8ml (0.06mol) propionyl chloride dropwise within 0.5 hours, and react at 5-20°C for 10 hours. Keeping the same temperature, 118ml of 10% HCl was added, and the reaction was continued for 2 hours. The organic phase was separated, the aqueous layer was extracted twice with 100ml ethyl acetate, the organic phases were combined, washed with saturated sodium bicarbonate solution, the ethyl acetate was evaporated under reduced pressure, and the fraction at 48-50°C / 670Pa was collected to obtain 15.8 grams, Yield 66.8%, purity 99.2% (GC).
Embodiment 2
[0024] 0.06mol butyryl chloride was reacted instead of propionyl chloride, and the same method as in Example 1 was collected to obtain 26.6 grams of ethyl butyryl acetate, with a yield of 72.6% and a purity of 99.6% (GC).
Embodiment 3
[0026] 0.06mol isobutyryl chloride replaces propionyl chloride and reacts, and the method identical with embodiment 1, collects the cut of 62-64 ℃ / 670Pa, obtains ethyl isobutyryl acetate 23.8 grams, productive rate 78.2%, purity 99.5% (GC) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com