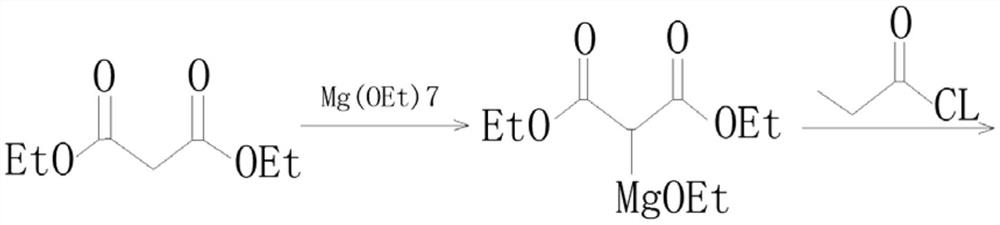
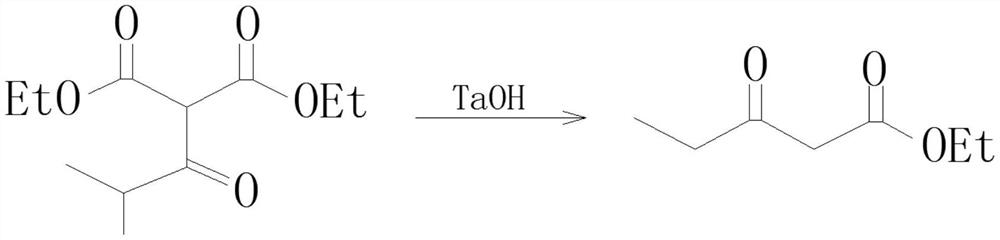
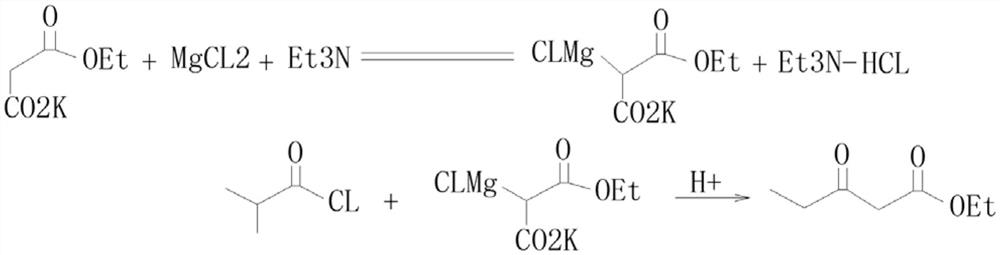
Preparation process of isobutyryl ethyl acetate
A technology for the preparation of ethyl isobutyrylacetate, which is applied in the field of medicine, can solve the problems of low productivity and low purity of ethyl isobutyrylacetate, and achieve the effects of solving low productivity, improving purity, and convenient production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] A kind of preparation technology of ethyl isobutyryl acetate, concrete steps are as follows:
[0023] (1), add 125mL ethyl acetate and 13.6g (80mmol) potassium monoethyl malonate to a three-necked flask, stir and cool to 0-5°C, then add 9.12g (96mol) anhydrous magnesium chloride and 27.8mL ( 0.2mol) triethylamine, be warming up to 35 ℃ in 0.5h and stir 6h at this temperature:
[0024] (2) Cool to 0°C, add 6mL (57mmol) isobutyryl chloride dropwise at 0-5°C for about 1 hour, then react at room temperature for 12 hours:
[0025] (3) Cool to 0°C, carefully add 70mL of 13% hydrochloric acid, keep the temperature not higher than 20°C during this process:
[0026] (4), separate the organic phase, extract the aqueous layer with toluene (40mL × 3), combine the organic phases, wash with saturated sodium bicarbonate solution until neutral, then wash with 25mL saturated brine, and evaporate the solvent under reduced pressure:
[0027] (5) The crude product was distilled under red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com