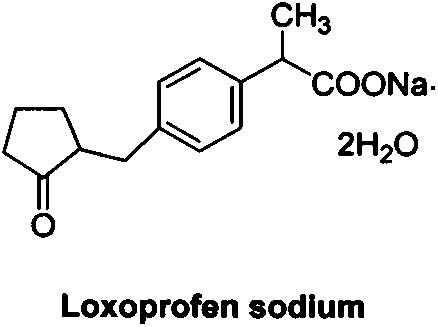
Industrial production method of high purity loxoprofen sodium dehydrate
A technology for loxoprofen sodium dihydrate and a production method, which is applied in the field of high-purity loxoprofen sodium dihydrate to achieve the effects of shortening reaction time, improving product quality and yield, and increasing conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of industrialized production method of high-purity loxoprofen sodium dihydrate:
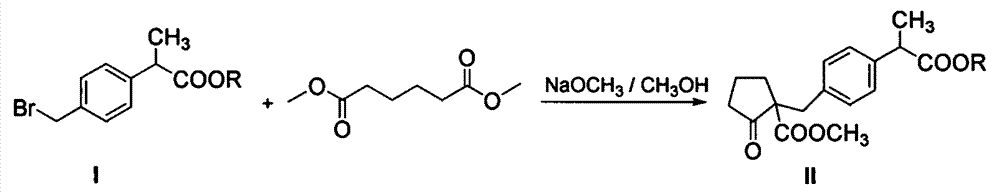
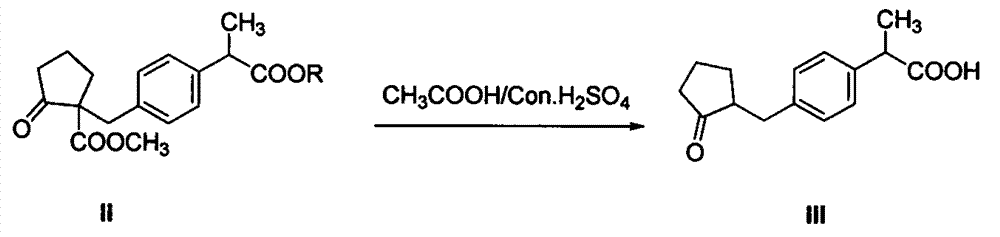
[0040] (1) Preparation of compound II:
[0041] Add 2Kg of dimethyl adipate to 6Kg of toluene, stir and cool to 0-10°C to obtain a toluene solution of dimethyl adipate, and then add dropwise 28% sodium methoxide to the toluene solution of dimethyl adipate Methanol solution 2Kg, and react at 0-10°C for 1 hour under heat preservation. After the reaction, heat up to 60-80°C and concentrate under normal pressure to obtain a ring-closing product reaction solution; then cool the obtained reaction solution to 10-15 After ℃, add 3Kg N, N-dimethylformamide (DMF), then directly dropwise add 2Kg of compound 2-(4-bromomethylphenyl) propionate shown in formula I in the reaction solution, dropwise process Maintain the temperature at 10-15° C., carry out the substitution reaction, and the reaction time is 3-5 hours. After the reaction is completed (normalized detection of the compound 2-(4-bromom...
Embodiment 2
[0053] A kind of industrialized production method of high-purity loxoprofen sodium dihydrate:
[0054] (1) Preparation of Compound II:
[0055] Add 2Kg of dimethyl adipate to 6Kg of toluene, stir and cool to 0-10°C to obtain a toluene solution of dimethyl adipate, and then add dropwise 28% sodium methoxide to the toluene solution of dimethyl adipate Methanol solution 2.2Kg, and react at 0-10°C for 1 hour under heat preservation, after the reaction, heat up to 60-80°C and concentrate under normal pressure to obtain a ring-closing product reaction solution; then cool the obtained reaction solution to 10-80°C After 15°C, 3Kg N,N-dimethylformamide (DMF) was first added, and then 2.1Kg of the compound 2-(4-bromomethylphenyl) propionate shown in formula I was directly added dropwise to the reaction solution, and then Adding process maintains temperature at 10~15 ℃, carries out substitution reaction, and the reaction time is 3~5h, after reaction is finished (with HPLC area normaliza...
Embodiment 3
[0062] A kind of industrialized production method of high-purity loxoprofen sodium dihydrate:
[0063] (1) Preparation of Compound II:
[0064] Add 2Kg of dimethyl adipate to 6Kg of toluene, stir and cool to 0-10°C to obtain a toluene solution of dimethyl adipate, and then add dropwise 28% sodium methoxide to the toluene solution of dimethyl adipate Methanol solution 1.9Kg, and react at 0-10°C for 1 hour under heat preservation, after the reaction, heat up to 60-80°C and concentrate under normal pressure to obtain a ring-closing compound reaction liquid; then cool the obtained reaction liquid to 10-10°C After 15°C, 3Kg N, N-dimethylacetamide was first added, and then 2.2Kg of the compound 2-(4-bromomethylphenyl) propionate shown in formula I was directly added dropwise to the reaction solution, and the dropping process maintained Temperature is 10~15 ℃, carries out substitution reaction, and the reaction time is 3~5h, after reaction finishes (with HPLC area normalization dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com