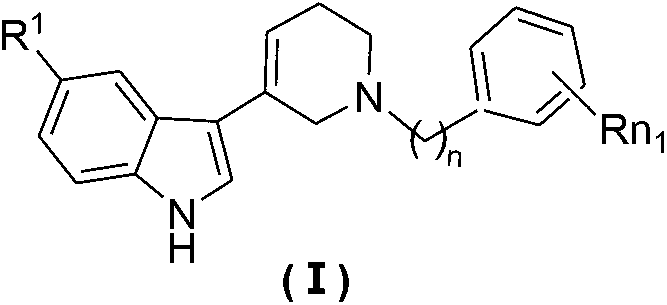
N-substituted benzyl tetrahydropyridine with indole and preparation method and application thereof
A technology of benzyl tetrahydropyridine linkage and indole, which is applied in the directions of organic chemistry, drug combination, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
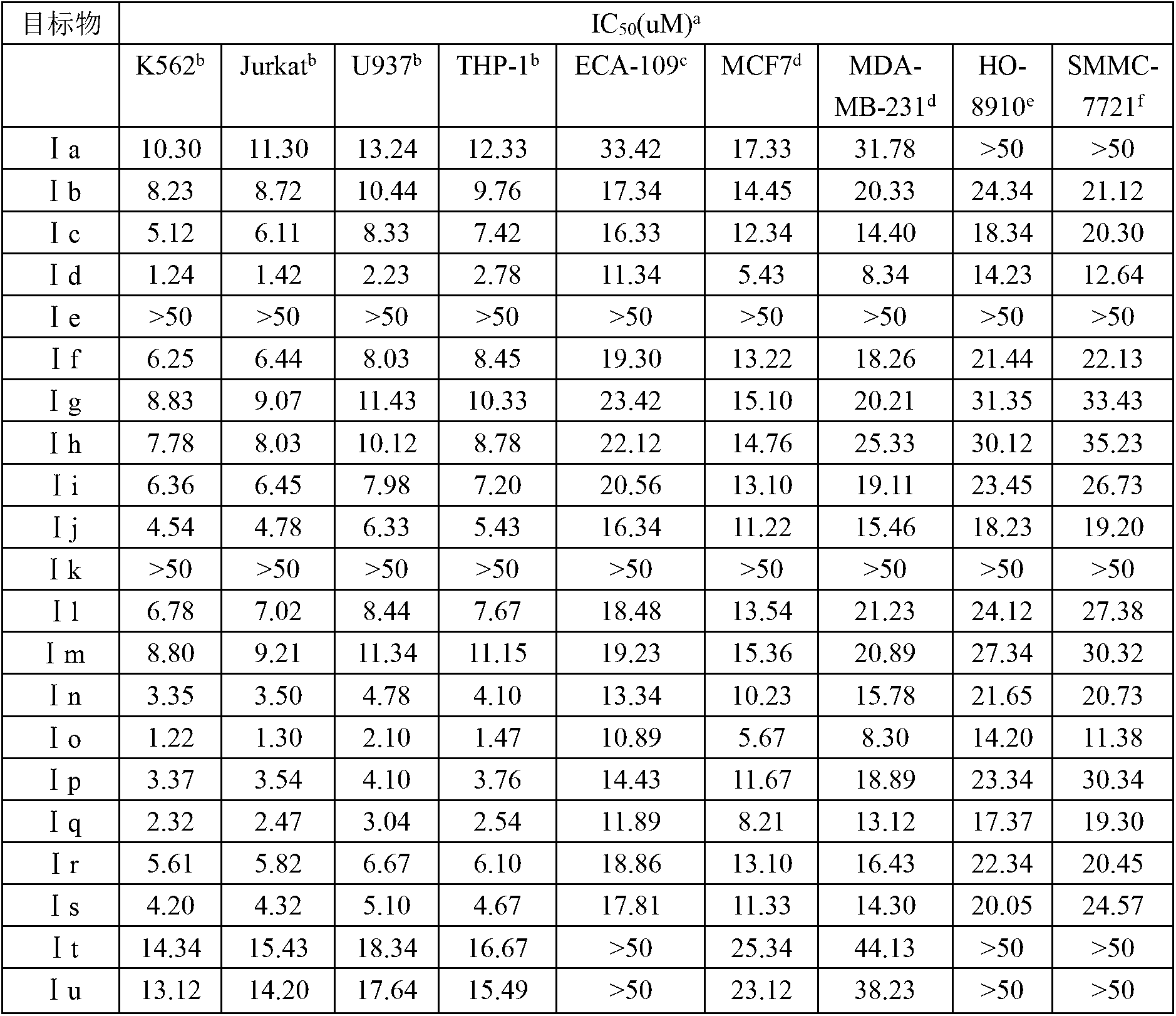
Embodiment 1~21
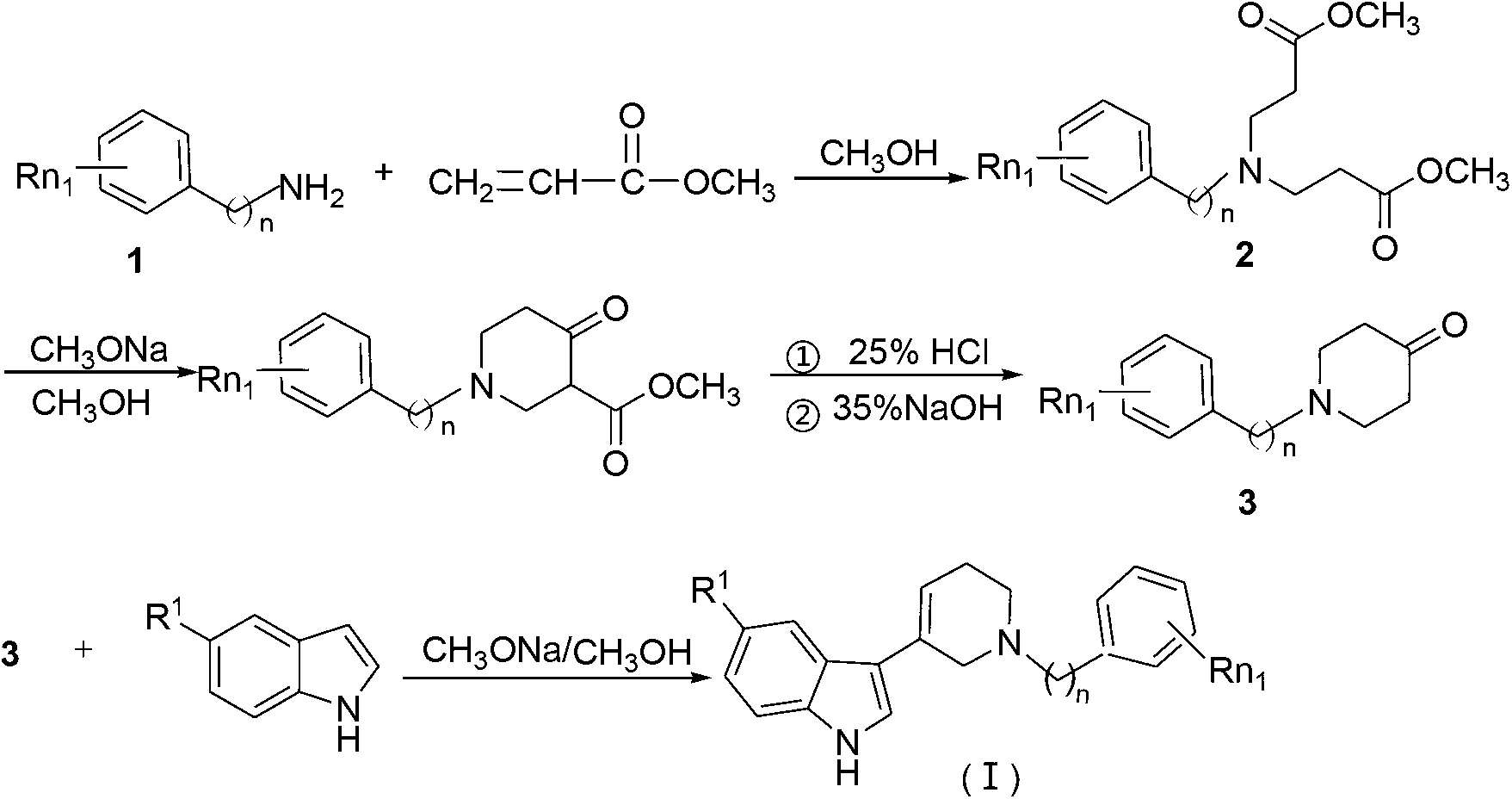
[0029] 1. Examples 1-21: The preparation method of N-substituted benzyl (ethyl)tetrahydropyridin-5-substituted indole of general formula (Ia-u):
[0030] 1) At room temperature, dissolve 0.04mol of the compound of formula (1) (substituted benzylamine or substituted phenylethylamine) in 4mL of methanol solution, and add dropwise to a mixed solution of 0.16mol of methyl acrylate and 7mL of methanol under stirring. Not exceeding 50°C. After the dropwise addition is completed, heat to reflux for 6-7 hours, the reflux temperature is 60-65°C, and the reaction progress is tracked by thin-layer chromatography (TLC). After the reaction is finished, methanol and unreacted methyl acrylate are recovered and distilled under reduced pressure to obtain a light yellow oily liquid as the compound of formula (2).
[0031] 2) Dissolve 0.04mol of the compound of formula (2) in 20mL of anhydrous toluene, and then drop into 15mL of anhydrous toluene and 0.122mol of metallic sodium solution under s...
Embodiment 1
[0034] Example 1 3-(N-benzyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indole (Ia)
[0035] Yield: 75%; yellow crystals; melting point: 180-182°C; 1 H NMR (400MHz, DMSO-d 6 )δ11.10(s, 1H), 7.79(d, J=8.0Hz, 1H), 7.56-6.78(m, 9H), 6.11(s, 1H), 3.67(s, 2H), 3.02(s, 2H ), 2.65 (t, J=5.5Hz, 2H), 2.49 (s, 2H); 820, 760cm -1 ;Anal.calcd.forC 20 h 20 N 2C% 83.30, H% 6.79, N% 9.71; Found: C% 82.15, H% 6.90, N% 9.80.
Embodiment 2
[0036] Example 2 3-(N-(4-chlorobenzyl)-1,2,3,6-tetrahydropyridin-4-yl)-1H-indole (Ib)
[0037] Yield: 72%; yellow crystals; melting point: 184-186°C; 1 H NMR (400MHz, DMSO-d 6 )δ11.02(s, 1H), 7.72(d, J=8.0Hz, 1H), 7.43-6.80(m, 8H), 6.02(s, 1H), 3.49(s, 2H), 3.02(d, J =2.7Hz, 2H), 2.59(t, J=5.6Hz, 2H), 2.48(s, 2H); IR(KBr): 3072, 3059, 2920, 2852, 1657, 1604, 1575, 1510, 1322, 1287 , 1103, 935, 815, 760cm -1 ;Anal.calcd.for C 20 h 19 ClN 2 C% 74.41, H% 5.93, N% 8.68; Found: C% 74.55, H% 6.01, N% 8.50.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com