Preparation method of substituted thiophene-3-one compound
A technology of ketone compounds and thiophene, which is applied in the field of preparation of substituted thiophene-3-ketone compounds, can solve the problems of poor environmental protection and high cost of raw materials, and achieve stable yields, increased total yields, and short synthetic routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] As described in the background art, the existing methods for preparing substituted thiophene-3-ones have the problems of high cost of raw materials and poor environmental protection. In order to solve the above technical problems, the invention provides a preparation method of substituted thiophene-3-one compounds, the preparation method comprising:
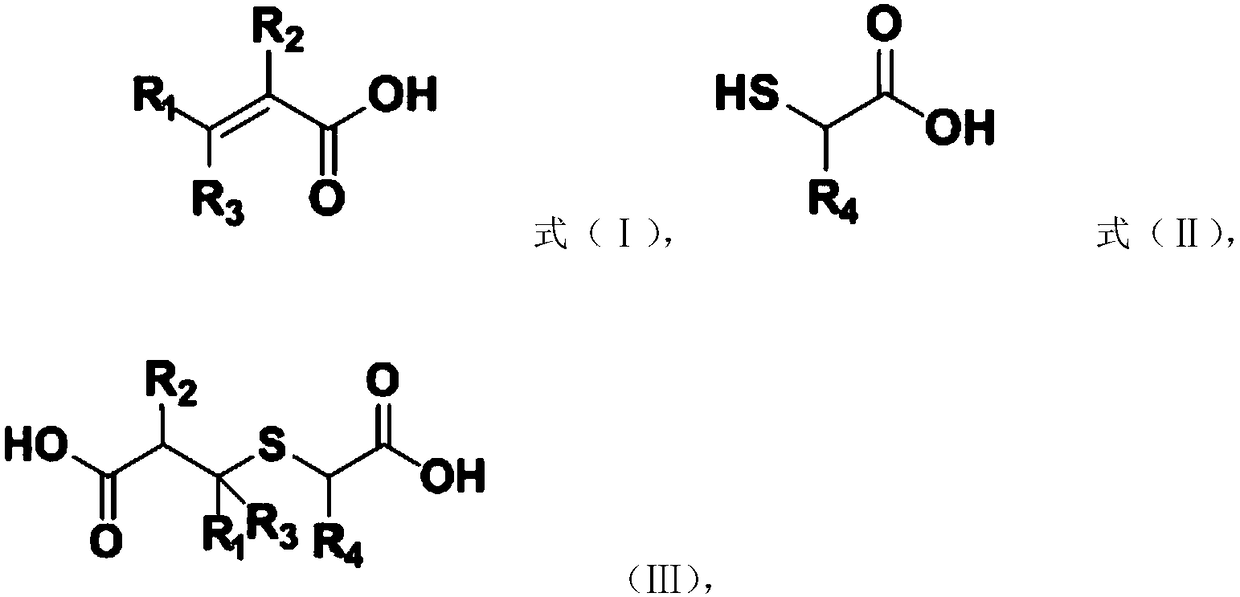
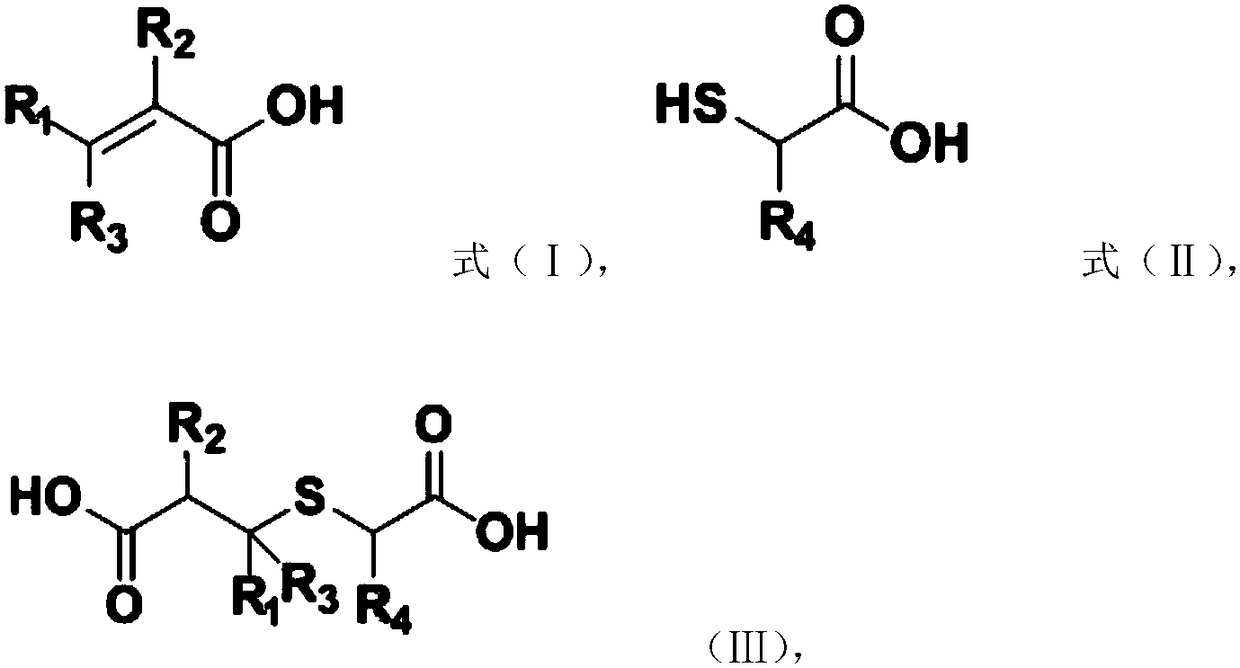
[0026] Michael addition reaction: Carry out Michael addition reaction between raw material A and raw material B to obtain the addition product, raw material A has the structure shown in formula (I), raw material B has the structure shown in formula (II), and the addition product has The structure shown in formula (Ⅲ),
[0027]
[0028] Among them, R 1 , R 2 , R 3 and R 4 independently selected from H, C 1 ~C 10 Alkyl or C 6 ~C 15 aryl; and
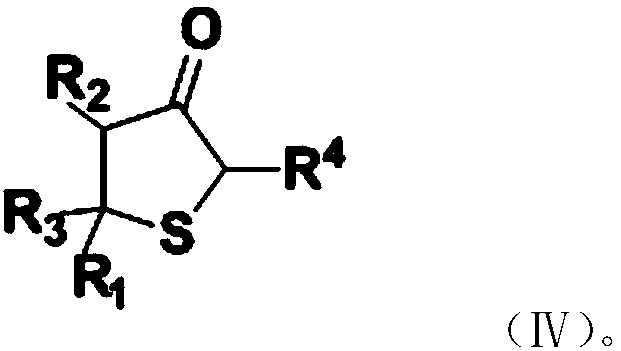
[0029] Dickman condensation reaction, the addition product is subjected to a Dickmann condensation reaction to obtain a substituted thiophene-3-one compound, and the substi...
Embodiment 1
[0052] FeCl 3 (1.625g, 0.01mol) was dissolved in acrylic acid (72g, 1mol) after being stirred and clarified, under nitrogen atmosphere, slowly added to thioglycolic acid (92g, 1mol) to obtain the system to be reacted;
[0053] Raise the temperature of the above-mentioned reaction system to 125 ° C, and stir for 1 h, sample HPLC, and obtain the addition product after the reaction of acrylic acid is completed;
[0054] The above-mentioned addition product system was heated up to 230° C. to carry out the Dickmann condensation reaction. After 1 hour, the reaction was completed, and the product system of the Dickmann condensation reaction was distilled to obtain 83 g of colorless liquid fraction and yellow liquid fraction product (tetrahydrothiophene-3 Ketone), the yield is 81wt%, and the purity is 95wt%.
[0055] The spectrogram data of the product are as follows:
[0056] 1 H NMR (500 MHz, DMSO) δ 3.23 (s, 2H), 3.01 (t, J = 7.1 Hz, 2H), 2.52 (t, J = 7.1 Hz, 2H).
[0057] EI-M...
Embodiment 2
[0059] The difference with Example 1 is: the catalyst is copper acetate.
[0060] After dissolving copper acetate (1.82g, 0.01mol) in acrylic acid (72g, 1mol) and stirring for clarification, under a nitrogen atmosphere, slowly add thioglycolic acid (92g, 1mol) to obtain a system to be reacted;
[0061] Raise the temperature of the above-mentioned reaction system to 125 ° C, and stir for 1 h, sample HPLC, and obtain the addition product after the reaction of acrylic acid is completed;
[0062] The above-mentioned addition product system was heated up to 230°C to carry out the Dickmann condensation reaction. After 1 hour, the reaction was completed, and the product system of the Dickmann condensation reaction was distilled to obtain a colorless distillate liquid and a yellow liquid distillate product (tetrahydrothiophene-3 ketone ), the yield of tetrahydrothiophene-3 ketone is 85wt%, and the purity is 97wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com