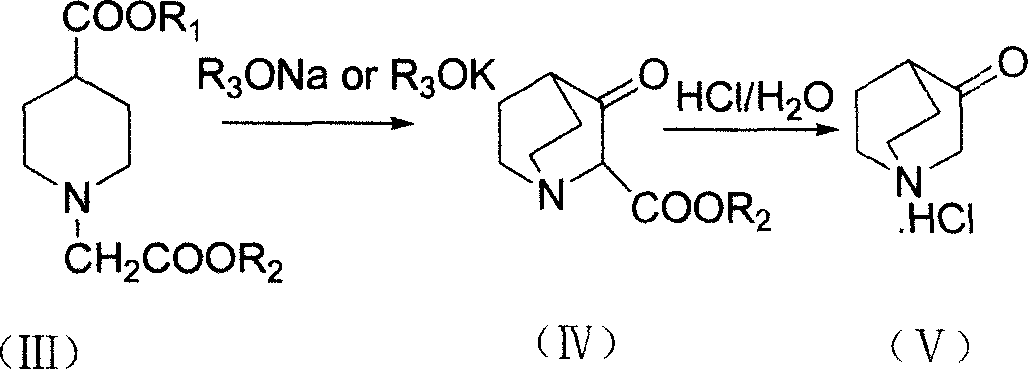
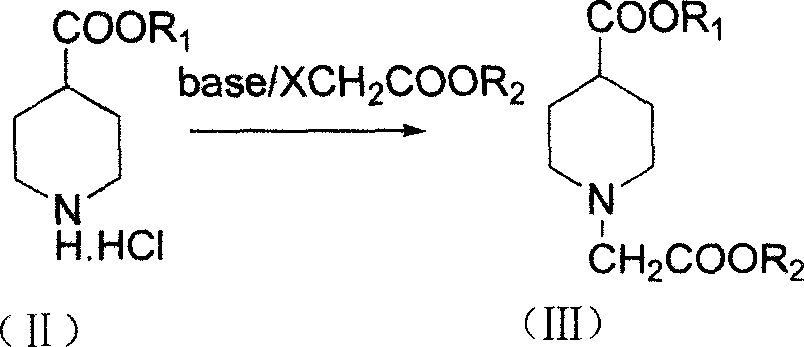Method of synthesizing 3-quininone hydrochlorate
A technology of quinine hydrochloride and synthesis method, which is applied in the direction of organic chemistry, etc., can solve problems such as inconvenient operation, and achieve the effects of simple method, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
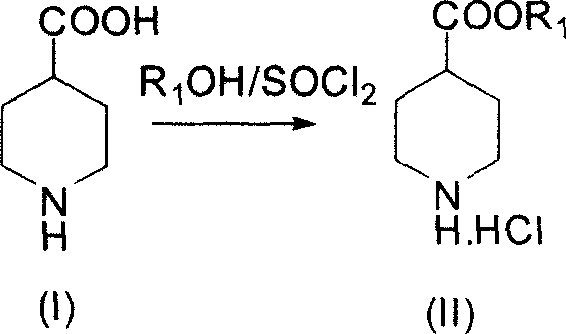
[0041] The synthetic method of 3-quininone hydrochloride is realized through the following steps:
[0042] 1) Preparation of ethyl 4-piperidinecarboxylate hydrochloride
[0043] Add 12.9g (0.1mol) of 4-piperidinecarboxylic acid and 150ml of absolute ethanol to a 250ml one-port bottle, and slowly add 30ml (0.3mol) of thionyl chloride dropwise at 0°C. The whole dropping process lasts for 1 hour. After the dropping, Raise the temperature and reflux for 6h, concentrate under reduced pressure to remove the solvent, add saturated NaOH solution to the residual solid to adjust to strong alkalinity, extract with 3×100ml chloroform, and dry the organic layer with calcium chloride; concentrate under reduced pressure to remove the solvent, and the residual Dissolve the solution in 100ml of absolute ethanol; pass hydrogen chloride gas into the ethanol, stir at 0°C for 0.5h, filter, and dry in vacuo to obtain 18.3g of white solid with a yield of 95%.
[0044] Mp: 142-145°C,
[0045] 1 H ...
Embodiment 2
[0056] The synthetic method of 3-quininone hydrochloride is realized through the following steps:
[0057] 1) Preparation of ethyl 4-piperidinecarboxylate hydrochloride
[0058] Add 12.9g (0.1mol) of 4-piperidinecarboxylic acid and 150ml of absolute ethanol to a 250ml one-port bottle, and slowly add 30ml (0.3mol) of thionyl chloride dropwise at 0°C. The whole dropping process lasts for 1 hour. After the dropping, Raise the temperature and reflux for 6h, concentrate under reduced pressure to remove the solvent, add saturated NaOH solution to the residual solid to adjust to strong alkalinity, extract with 3×100ml chloroform, and dry the organic layer with calcium chloride; concentrate under reduced pressure to remove the solvent, and the residual Dissolve the solution in 100ml of absolute ethanol; pass hydrogen chloride gas into the ethanol, stir at 0°C for 0.5h, filter, and dry in vacuo to obtain 18.3g of white solid with a yield of 95%.
[0059] MP: 142-145°C
[0060] 1 H N...
Embodiment 3
[0070] The synthetic method of 3-quininone hydrochloride is realized through the following steps:
[0071] 1) Preparation of ethyl 4-piperidinecarboxylate hydrochloride
[0072] Add 12.9g (0.1mol) of 4-piperidinecarboxylic acid and 150ml of absolute ethanol to a 250ml one-port bottle, and slowly add 30ml (0.3mol) of thionyl chloride dropwise at 0°C. The whole dropping process lasts for 1 hour. After the dropping, Raise the temperature and reflux for 6h, concentrate under reduced pressure to remove the solvent, add saturated NaOH solution to the residual solid to adjust to strong alkalinity, extract with 3×100ml chloroform, and dry the organic layer with calcium chloride; concentrate under reduced pressure to remove the solvent, and the residual Dissolve the solution in 100ml of absolute ethanol; pass hydrogen chloride gas into the ethanol, stir at 0°C for 0.5h, filter, and dry in vacuo to obtain 18.3g of white solid with a yield of 95%.
[0073] MP: 142-145°C
[0074] 1 H N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com