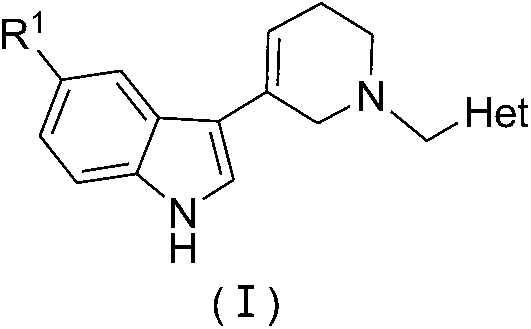
N-heterocyclic methyl tetrahydropyridine-5-substituted indole and preparation method and application thereof
A technology of heterocyclic methyltetrahydropyridine linkage and indole, which can be applied in the fields of organic chemistry, drug combination, antitumor drugs, etc., and can solve the problem of few drug candidates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~12
[0029] Embodiments 1-12 The preparation method of general formula (I) N-heterocyclic methyltetrahydropyridine-5-substituted indole is as follows:
[0030] At room temperature, dissolve the substituted heterocyclic primary amine (0.04mol) (1) in 4mL of methanol solution, add dropwise into the mixed solution of methyl acrylate (0.16mol) and methanol (7mL) under stirring, the temperature of the reaction system does not exceed 50 ℃. After the dropwise addition is completed, heat to reflux for another 7 to 8 hours, the reflux temperature is 65 to 75°C, and the reaction progress is tracked by thin layer chromatography (TLC). After the reaction, methanol and unreacted methyl acrylate were recovered and distilled under reduced pressure to obtain light yellow oily liquid (2).
[0031] Dissolve (2) (0.04mol) in anhydrous toluene (20mL) mixture, add dropwise under stirring, into anhydrous toluene (15mL) and metal sodium (0.122mol) solution, after dropwise addition, reflux for 6-8h. Dur...
Embodiment 13
[0034] Example 13-(N-(2-pyridylmethyl)-1,2,3,6-tetrahydropyridin-4-yl)-1H-indole (Ia)
[0035] Yield: 60%; yellow crystals; melting point: 160-162°C; 1 H NMR (400MHz, DMSO-d 6 )δ11.10(s, 1H), 7.79(d, J=8.0Hz, 1H), 7.56-6.78(m, 8H), 6.11(s, 1H), 3.67(s, 2H), 3.02(s, 2H ), 2.65 (t, J=5.5Hz, 2H), 2.49 (s, 2H); 820, 760cm -1 ;Anal.calcd.for C 19 h 19 N 3 C% 78.86, H% 6.62, N% 14.52; Found: C% 78.75, H% 6.90, N% 14.60.
Embodiment 23
[0036] Example 23-(N-(3-pyridylmethyl)-1,2,3,6-tetrahydropyridin-4-yl)-1H-indole (Ib)
[0037] Yield: 60%; yellow crystals; melting point: 166-168°C; 1 H NMR (400MHz, DMSO-d 6)δ11.08(s, 1H), 7.71(d, J=8.0Hz, 1H), 7.52-6.73(m, 8H), 6.13(s, 1H), 3.66(s, 2H), 3.01(s, 2H ), 2.62(t, J=5.5Hz, 2H), 2.49(s, 2H); IR(KBr): 3072, 3067, 2918, 2842, 1657, 1608, 1580, 1510, 1310, 1293, 1102, 940, 820, 760cm -1 ;Anal.calcd.for C 19 h 19 N 3 C% 78.86, H% 6.62, N% 14.52; Found: C% 78.70, H% 6.96, N% 14.62.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com