Preparation and application of beta-diketone boron fluoride fluorescent dye adopting first-class coumarin as framework
A technology of diketone boron fluoride and fluorescent dyes, which is applied in the field of β-diketone boron fluoride fluorescent dyes and its preparation and application, can solve the problems of small Stocks shift and limit the better application of coumarin, and achieve optical Stable properties, easy derivatization, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0041] [Example 2] Synthesis of a fluorescent dye formula I compound (i.e. I1) of a β-diketone boron fluoride with coumarin as a skeleton, wherein R1=R2=R3=R4=diethylamino, and the reaction The formula is as follows:
[0042]
[0043] Preparation I1: Dissolve 0.3g (0.597mmol) of intermediate V1 in anhydrous dichloromethane, add 0.18g (1.791mmol) of triethylamine and stir at room temperature for 30min, then add 0.252g (1.791mmol) of boron trifluoride diethyl ether, stirred the reaction mixture and heated to reflux for 4 hours, extracted three times with water and dichloromethane, separated the organic layer with a separating funnel, collected the organic layer, dried over anhydrous sodium sulfate, evaporated the solvent in a rotary evaporator, and washed with dichloromethane After recrystallization and suction filtration, 0.1 g of solid was obtained, namely product I1, with a yield of 56%.
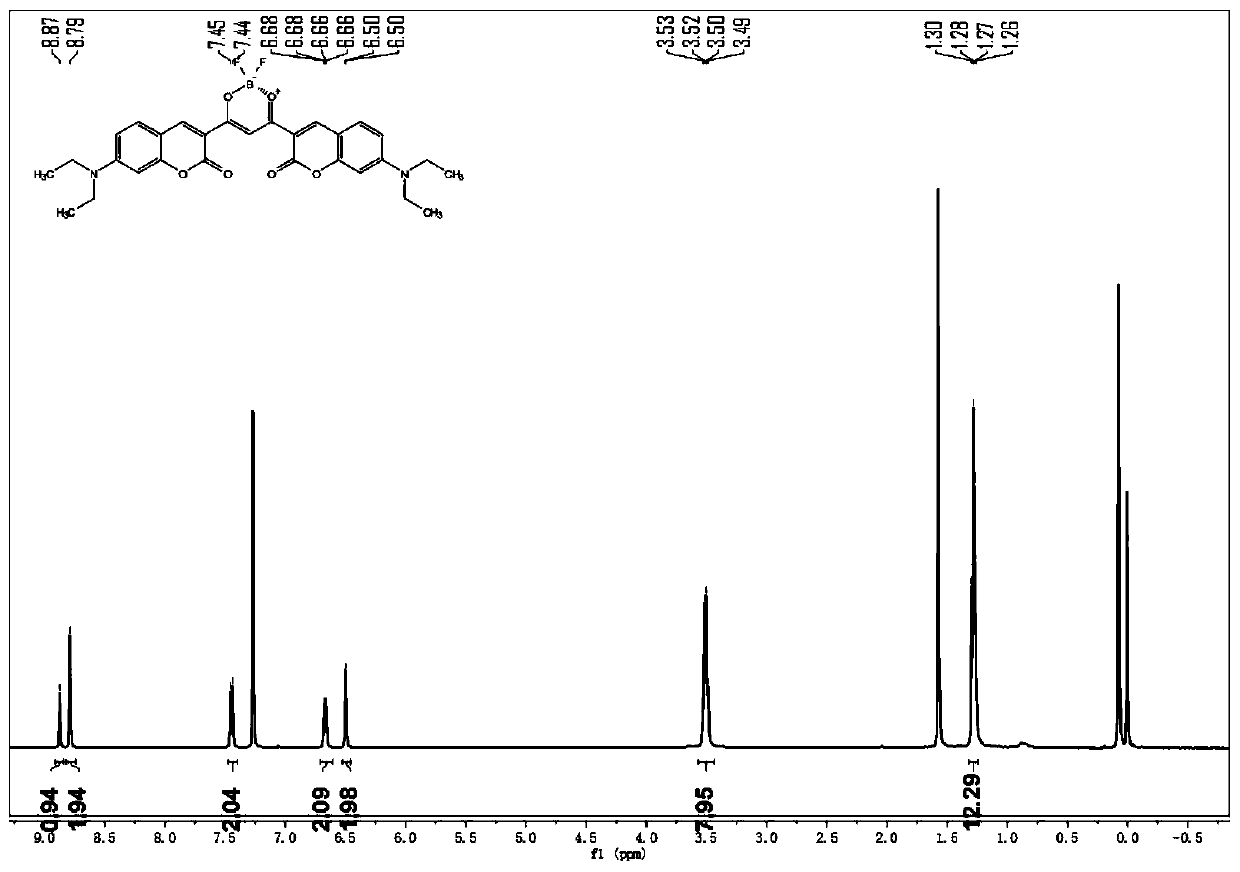
[0044] figure 1 It is the H NMR spectrum of the compound prepared in Example 2. S...
Embodiment 4
[0050] [Example 4] A kind of synthesis of fluorescent dye compound II of β-diketone boron fluorides with coumarin as the skeleton, the reaction formula is as follows:
[0051]
[0052] Take 0.3g (0.545mmol) of compound VIII and dissolve it in anhydrous dichloromethane, then add 0.165g (1.64mmol) of triethylamine and stir at room temperature for 30min, then add 0.165g (1.64mmol) of boron trifluoride ether, and stir the reaction The mixture was extracted three times with water and dichloromethane for 4 hours, and the organic layer was separated with a separating funnel. After the organic layer was collected and dried on anhydrous sodium sulfate, the solvent was evaporated in a rotary evaporator, recrystallized with dichloromethane, and 0.12g was obtained by suction filtration. The solid is the product II, and the yield is 60%.
[0053] Figure 4 It is the H NMR spectrum of the compound prepared in Example 4. Structure: 1 H NMR (500MHz, TFA) δ7.68(s, 2H), 6.25(s, 2H), 2.43(...
Embodiment 5
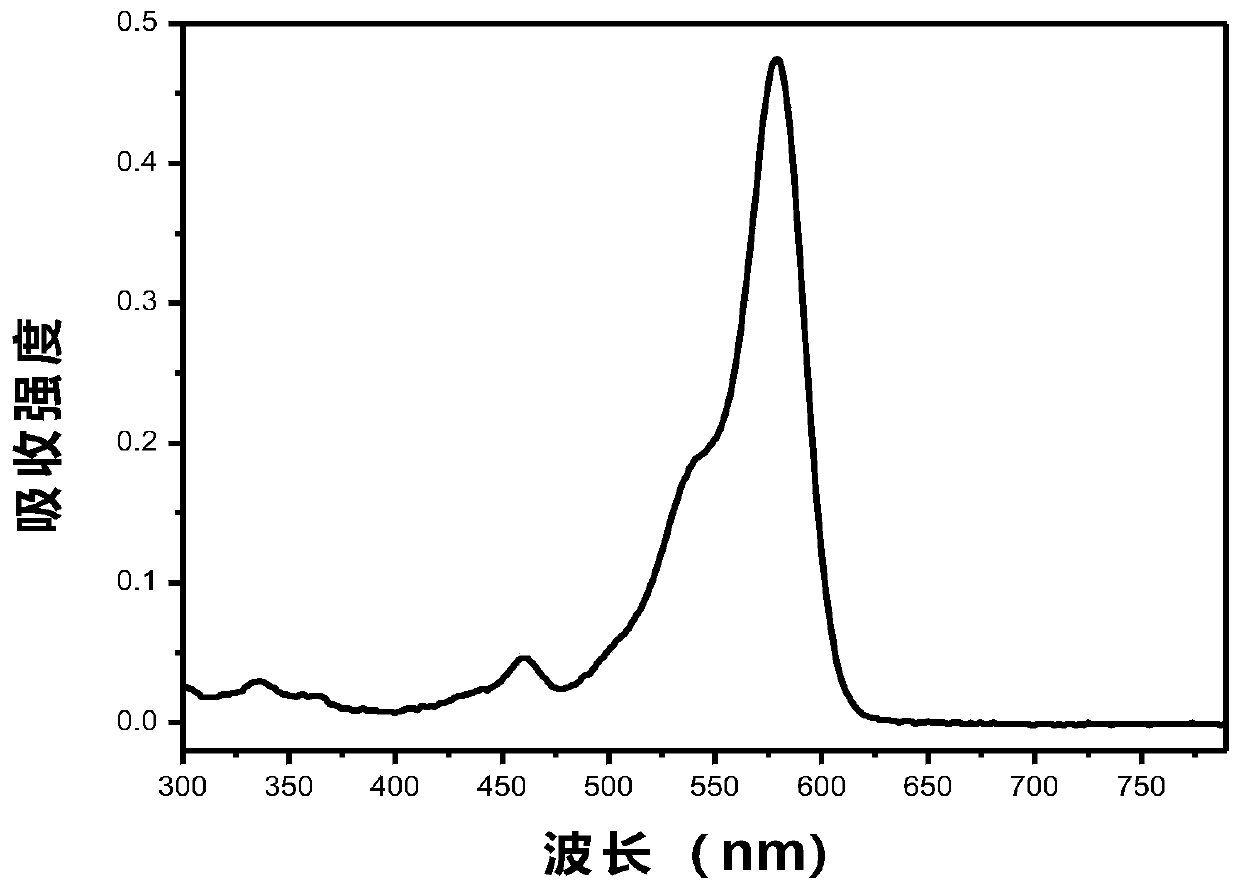
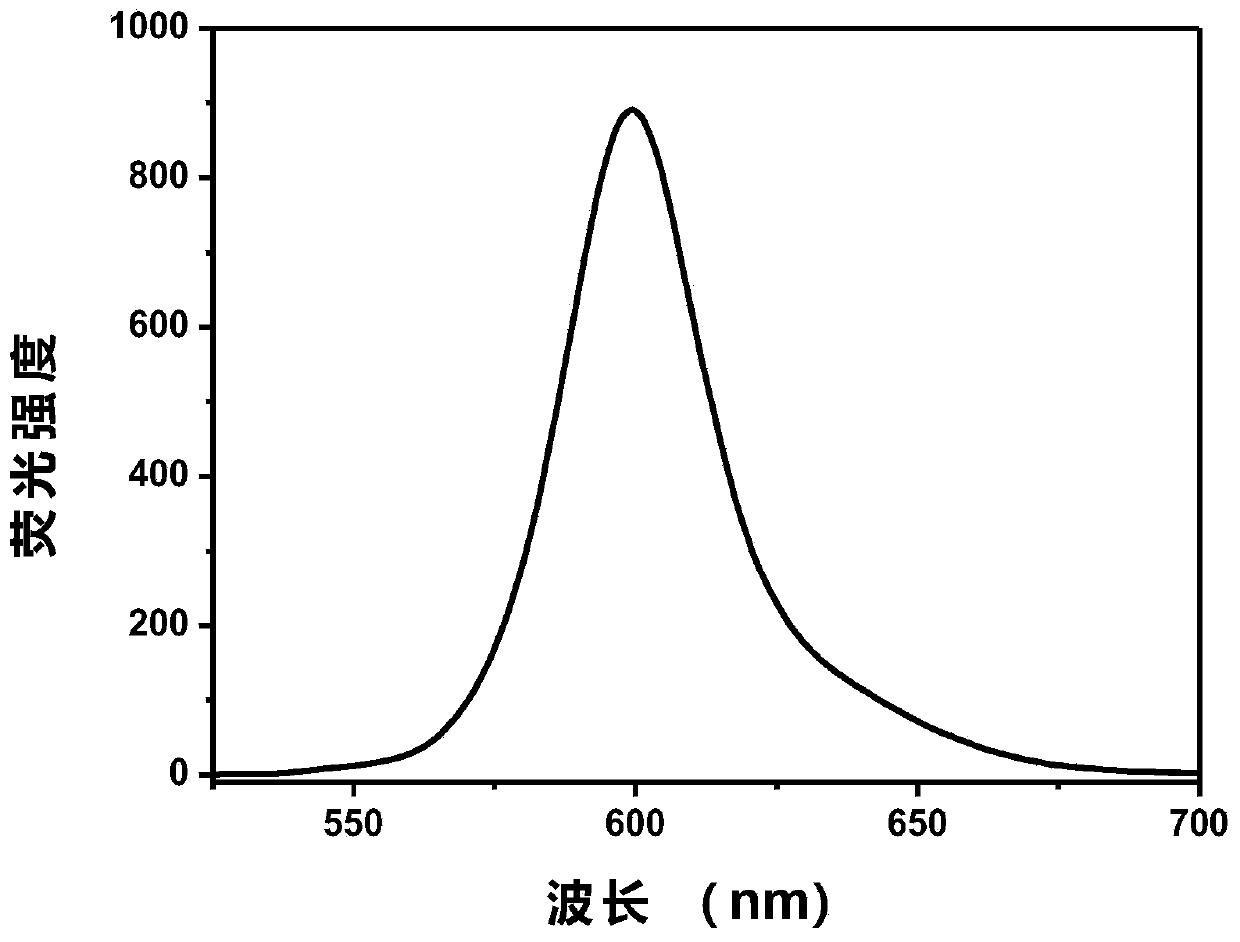
[0055] [Example 5] Compound I1 is formulated with toluene, dichloromethane, tetrahydrofuran, and ethyl acetate to form a molar concentration of 10 -5 mol / L solution, the UV absorption spectrum and fluorescence emission spectrum were measured, and the basic photophysical related data are shown in Table 1.
[0056] Compound II was formulated with toluene, dichloromethane, tetrahydrofuran, and ethyl acetate to make a molar concentration of 10 -5 mol / L solution, the UV absorption spectrum and fluorescence emission spectrum were measured, and the basic photophysical related data are shown in Table 2.
[0057] Table 1 compound formula I1 relevant photophysical data in each solvent
[0058]
[0059] The results in Table 1 show that the maximum absorption wavelengths of dye I1 in toluene, dichloromethane, tetrahydrofuran, and ethyl acetate are 579, 597, 589, and 584, and the maximum emission wavelengths are 600, 630, 615, and 613, respectively. There are better fluorescence quant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com