Method for preparing scutellarin
An aglycone and breviscapine technology, which is applied in the direction of organic chemistry and the like, can solve the problems of difficult industrial application, difficult to obtain in large quantities, cumbersome process operation, etc., and achieves high industrial application value and economic value, simple and convenient operation, and good purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
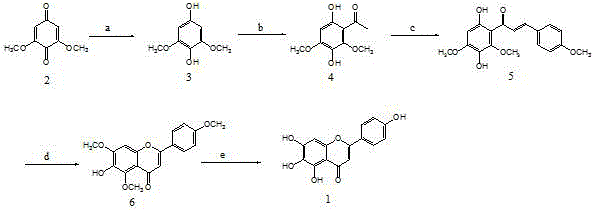
[0020] (1) Synthesis of Compound 3: Weigh 16.8g (0.10mol) of 2,6-dimethoxy-p-benzoquinone (Compound 2) into a 250mL round-bottomed flask, add 10% sodium hydrosulfite aqueous solution 100mL (the molar amount of sodium hydrosulfite is 1 times the molar amount of compound 2), stirred at room temperature for about 3 hours, then filtered, washed the filter cake with a small amount of water, pressed dry, and dried under vacuum to obtain an off-white powdery solid, namely Compound 3; weighing: 17.0 g, yield: 100%. 1 HNMR (DMSO): δ5.73(s,2H), δ5.05(s,1H), δ3.77(s, 6H).
[0021] (2) Synthesis of Compound 4: Weigh 17.0g (0.10mol) of Compound 3 into a 250mL reaction bottle, add 100mL of chloroform and 25mL of acetic anhydride, then slowly add about 20mL of boron trifluoride ether solution under stirring at room temperature ( Boron trifluoride is 0.2mol); slowly heat the reaction mixture to about 90°C and react for about 10 hours; let it cool, add water dropwise, slowly heat up and evapo...
Embodiment 2
[0026] (1) Synthesis of Compound 3: Weigh 16.8g (0.10mol) of 2,6-dimethoxy-p-benzoquinone (Compound 2) into a 250mL round bottom flask, add 100mL industrial methanol and no less than 3.6g ( 0.15mol) of magnesium metal, heat the mixture to 60°C under stirring for about 5-6 hours, filter, wash with a little fresh methanol, combine the filtrate, evaporate methanol under reduced pressure to obtain an off-white powdery solid, the compound 3. Colorless crystals that can be recrystallized from a methanol-water mixed solvent, dried, weighed: 16.0 g, yield: 94.0%. 1 HNMR (DMSO): same as step (1) of Example 1;
[0027] (2) Synthesis of Compound 4: Weigh 17.0g (0.10mol) of Compound 3 into a 100mL reaction bottle, add 35mL of acetic acid, then slowly add about 10mL of phosphorus oxychloride under stirring at room temperature; slowly heat the reaction mixture to React at about 100°C for about 8 hours. Allow the reaction mixture to cool down and add dropwise to 200mL of water. The aqueous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com