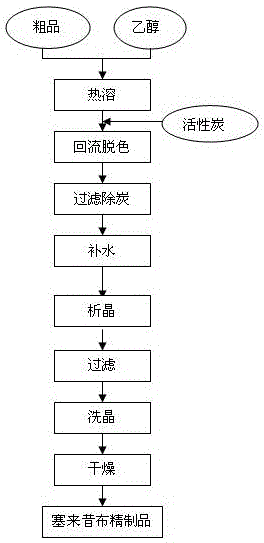
Celecoxib preparation method
A technology of celecoxib and ethanol, applied in the field of drug synthesis, can solve the problems of low yield and poor purity, and achieve the effects of high purity, uniform distribution and fine particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0042] The preparation of embodiment 1 ethyl trifluoroacetate
[0043]
[0044] Add 300mL of trifluoroacetic acid in batches to 1.4L of absolute ethanol, then add 100g of D072 strongly acidic cation exchange resin as a catalyst, heat and stir under reflux for 8 hours, filter out the resin under reduced pressure, and rectify to obtain 340mL of ethyl trifluoroacetate with a boiling point of 60 ~62°C, the yield was 94.2%.
Embodiment 2
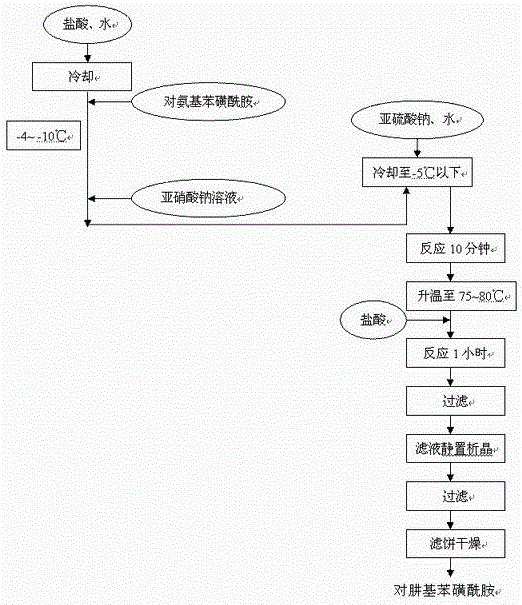
[0045] The preparation of embodiment 2 p-hydrazinobenzenesulfonamide hydrochloride
[0046]
[0047] P-aminobenzenesulfonamide is first reacted with sodium nitrite to obtain diazonium salt, and then reduced by sodium nitrite to obtain hydrazine.
[0048] Table 1, preparation of feed intake and proportioning of p-hydrazinobenzenesulfonamide hydrochloride
[0049]
[0050] Operation process:
[0051] A. Put (41kg hydrochloric acid and 60kg purified water) mixture into a 200L (208#) reaction tank, put 20kg p-aminobenzenesulfonamide under stirring, and cool down to below -4~-10°C. spare.
[0052] B. Weigh 8.84kg of sodium nitrite in a 50L barrel, add 24.8kg of purified water, and stir until completely dissolved.
[0053] C. Drop the sodium nitrite solution into the 208# reaction tank, control the temperature during the process at -4~-10°C, and stir for 10 minutes after the drop is completed.
[0054] D. Add 96.8kg of purified water into a 500L (204#) reaction tank, add 3...
Embodiment 3
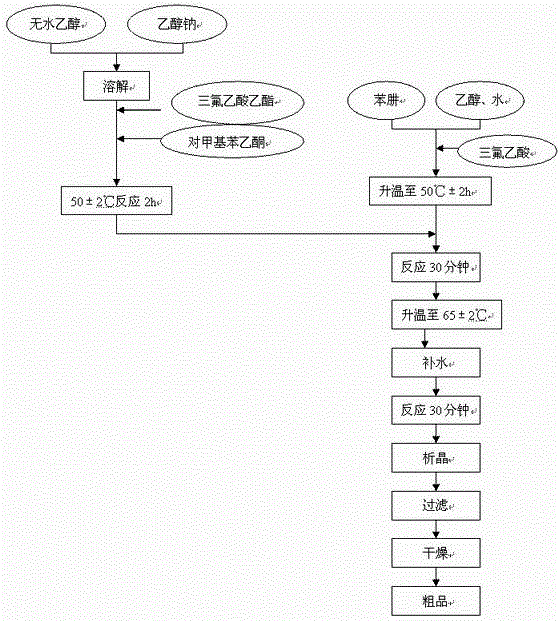
[0068] Example 3 Preparation of 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione
[0069]
[0070] Table 5, the preparation of 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione feeding and ratio
[0071]
[0072] Operation process:
[0073] A. Put 47.6kg of absolute ethanol into 208# reaction tank (need to be dried), add 7.62kg of sodium ethoxide, and stir until completely dissolved.
[0074] B. Control the temperature below 40°C, add 16.1 kg of ethyl trifluoroacetate, and stir for 5 minutes. Add 11.42 kg of p-methylacetophenone, raise the temperature to 50±2°C, and keep stirring for 2 hours. spare.
[0075] Optimization of reaction conditions:
[0076] Table 6. Research on the reaction conditions for the preparation of 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione
[0077]
[0078] According to the analysis results, if the reaction temperature is lower than 40°C, the reaction of the raw materials will not be completed; if the reaction temperature is high...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com