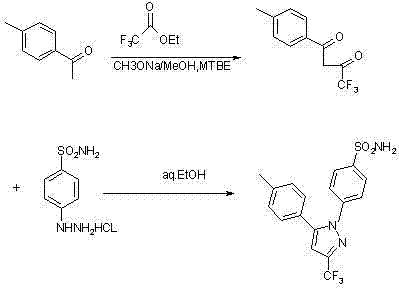
Synthesis method of celecoxib
A synthesis method and a condensation reaction technology are applied in the field of preparation of celecoxib, which can solve the problems of affecting product quality and yield, low celecoxib yield and the like, and achieve a sufficient reaction, improved competitiveness and less impurities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione
[0024] Put 400ml of toluene and 25g of sodium hydride into a 1000ml four-necked bottle successively, stir, raise the temperature to 60~65°C, add 40g of p-methylacetophenone dropwise, and simultaneously add 50g of ethyl trifluoroacetate dropwise. After dropping, keep warm at 60-65°C for 1 hour, cool to 30°C, add 120ml of 15% hydrochloric acid dropwise, let stand and separate after dropping, and evaporate the organic layer to dryness under reduced pressure to obtain the residue 1-(4-methyl 65.9 g of phenyl)-4,4,4-trifluoro-1,3-butanedione, yield 96%.
Embodiment 2
[0026] Preparation of 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione
[0027] Put 400ml of toluene and 25g of sodium hydrogen into a 1000ml four-necked bottle successively, stir, control the temperature to 20~25°C, add 40g of p-methylacetophenone dropwise, and simultaneously add 50g of ethyl trifluoroacetate dropwise. After dripping, keep it warm at 40-45°C for 5 hours, cool to 30°C, add 120ml of 15% hydrochloric acid dropwise, let stand to separate after dripping, evaporate the organic layer to dryness under reduced pressure, and add 200ml of petroleum ether to the residue to crystallize 1- (4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione 62.3g, yield 91%.
Embodiment 3
[0029] Preparation of 1-(4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione
[0030] Put 400ml of toluene and 40g of potassium hydride into a 1000ml four-neck bottle successively, stir, control the temperature to 5~10°C, add 40g of p-methylacetophenone dropwise, and simultaneously add 50g of ethyl trifluoroacetate dropwise. After dropping, keep warm at 30-35°C for 6 hours, cool to 30°C, add 120ml of 15% hydrochloric acid dropwise, let stand and separate after dropping, evaporate the organic layer to dryness under reduced pressure, and add 200ml of petroleum ether to the residue to crystallize 1- (4-methylphenyl)-4,4,4-trifluoro-1,3-butanedione 59g, yield 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com