Preparation method of pitavastatin calcium
A technology of pitavastatin calcium and intermediates, which is applied in the field of preparation of hypolipidemic drugs, can solve problems such as difficulty in large-scale production, difficult purification and processing of intermediates, and achieves reasonable cost, simple and feasible synthesis route, and is suitable for large-scale production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
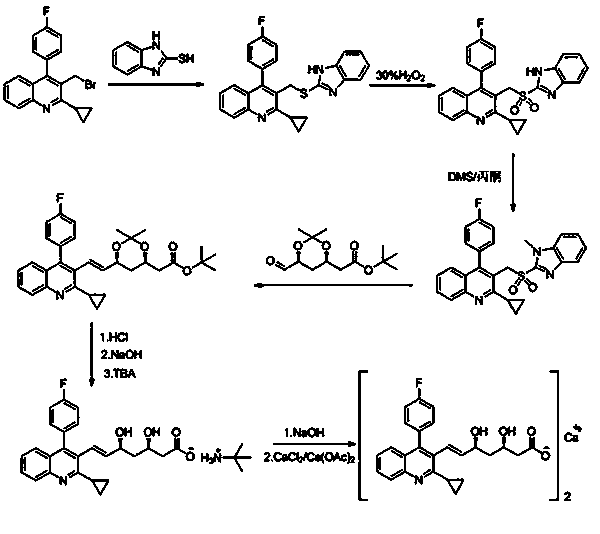
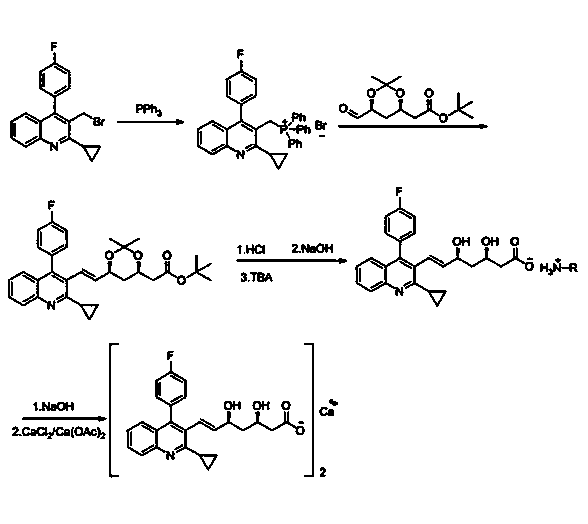
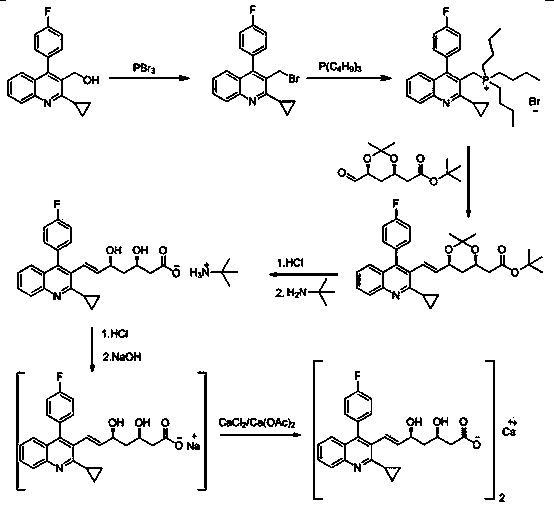
Image
Examples
Embodiment 1
[0027] Preparation of Intermediate I: In 150mL of dichloromethane, add 50g of 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol and 11.8g of potassium carbonate, stir, and cool down to 5°C; 13.8g of phosphorus tribromide was dissolved in 50mL of dichloromethane solution, and then slowly added dropwise to the above-mentioned reaction solution; after the drop was completed, the temperature was slowly raised to room temperature for reaction, and the reaction was monitored by TLC; after the reaction was completed, the reaction solution was poured into 800mL of ice water, Stir, separate the organic phase, extract the aqueous layer with 200 mL of dichloromethane, combine the organic phases, wash with 150 mL of saturated brine, dry over anhydrous sodium sulfate, evaporate to dryness under reduced pressure, add 200 mL of ether to the residue for beating, suction filter, and dry Dry to give 52.7 g of solid product. Yield: 87%.
[0028] Preparation of intermediate Ⅱ: put 45g of...
Embodiment 2
[0033] Preparation of intermediate Ⅰ: In 100mL of tetrahydrofuran, add 35g of 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol and 8.2g of potassium carbonate, stir, and cool down to 15°C; 16.1g Phosphorus tribromide was dissolved in 30mL tetrahydrofuran solution, and then slowly added dropwise to the above reaction solution; after dropping, the temperature was slowly raised to room temperature for reaction, and the reaction was monitored by TLC; after the reaction, the reaction solution was poured into 500mL ice water, stirred, and The organic phase and the aqueous layer were extracted with dichloromethane (150mL×2), washed with 150mL saturated brine, dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure, 100mL ether was added to the residue for slurry, suction filtered, and dried to obtain 38.2 solid product. Yield: 90%.
[0034] Preparation of Intermediate II: Put 30g of Intermediate I in acetonitrile, stir to dissolve, cool down to 0°C,...
Embodiment 3
[0039] Preparation of Intermediate I: In 100mL of dichloromethane, add 20g of 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinemethanol and 4.7g of potassium carbonate, stir, and cool down to 15°C; 14.7g of phosphorus tribromide was dissolved in 50mL of dichloromethane solution, and then slowly added dropwise to the above-mentioned reaction solution; after dropping, the temperature was slowly raised to room temperature for reaction, and the reaction was monitored by TLC; after the reaction was completed, the reaction solution was poured into 800mL of ice water, Stir, separate the organic phase, extract the aqueous layer with 200 mL of dichloromethane, combine the organic phases, wash with 200 mL of saturated brine, dry over anhydrous sodium sulfate, evaporate to dryness under reduced pressure, add 120 mL of ether to the residue for beating, suction filter, and dry Drying yielded 22.3 g of solid product. Yield: 92%.
[0040] Preparation of intermediate Ⅱ: Put 20g of intermediat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com