Method for separating and determining pitavastatin and its optical isomer by means of liquid chromatography
A technology for optical isomers and determination methods, applied in the field of analytical chemistry, can solve unreliable problems, achieve the effects of improving symmetry, ensuring stability, and controlling quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Instruments and Conditions
[0032] American Agilent 1100 high-performance liquid chromatography system and workstation; automatic sampling; CHIRALPAK-AD chiral chromatography column (250mm×4.6mm) as the separation column; UV detection wavelength: 245nm; mobile phase: n-hexane-ethanol solution (containing 1.0% trifluoroacetic acid) (92:8) as mobile phase; column temperature 40°C. The injection volume was 10 μl.
[0033] Experimental procedure
[0034] Take about 25mg of pitavastatin calcium racemate, put it in a 50ml measuring bottle, add ethylene glycol dimethyl ether to dissolve and dilute to the mark, shake well, and use it as the test solution.
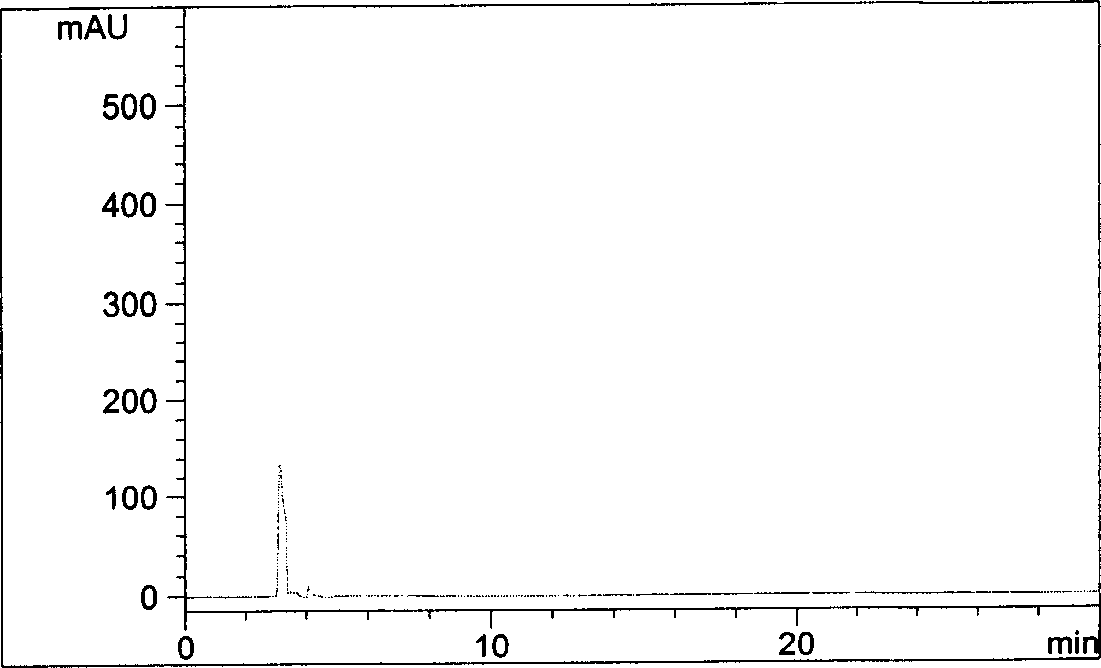
[0035] Get reagent blank solution and need testing solution respectively, carry out high performance liquid chromatography analysis according to above-mentioned conditions, record chromatogram, the result sees figure 1 , figure 2 .
[0036] figure 2 The chromatographic peak with a retention time of 17.222 minutes is...
Embodiment 2
[0038] Take about 25mg of pitavastatin calcium, put it in a 50ml measuring bottle, add ethylene glycol dimethyl ether to dissolve and dilute to the mark, shake well, and use it as the test solution.
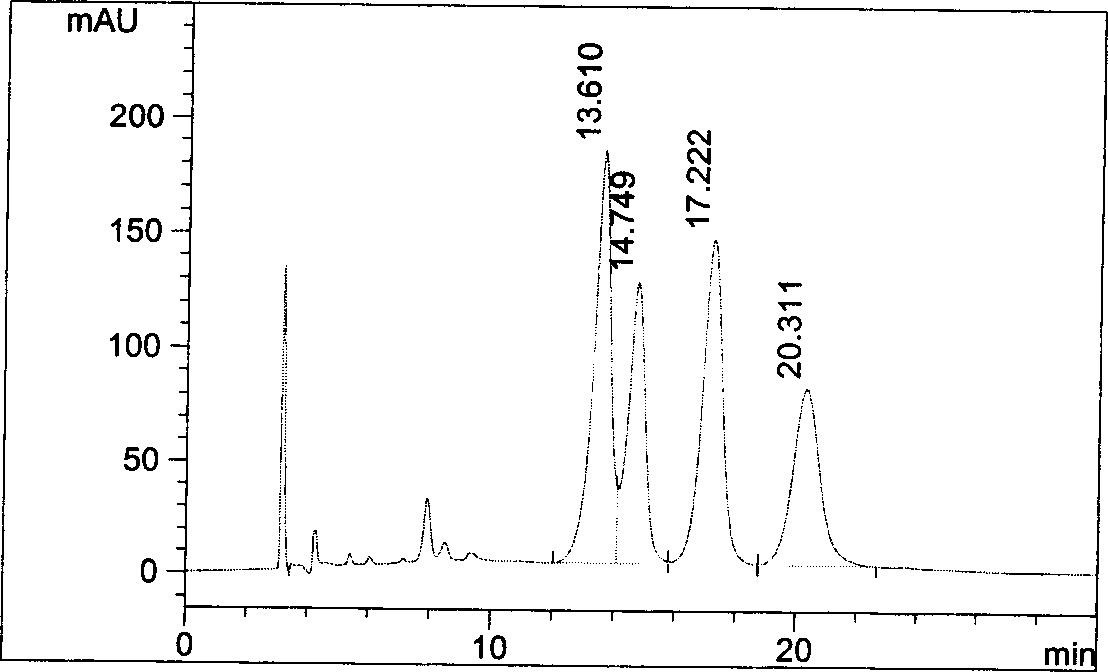
[0039] Get need testing solution, carry out high performance liquid chromatography analysis according to the condition of embodiment 1, record chromatogram, the results are shown in image 3 .
[0040] image 3 It is proved that the optical purity of pitavastatin calcium meets the requirements of raw materials, and this method can be used for quality monitoring of pitavastatin calcium.
Embodiment 3
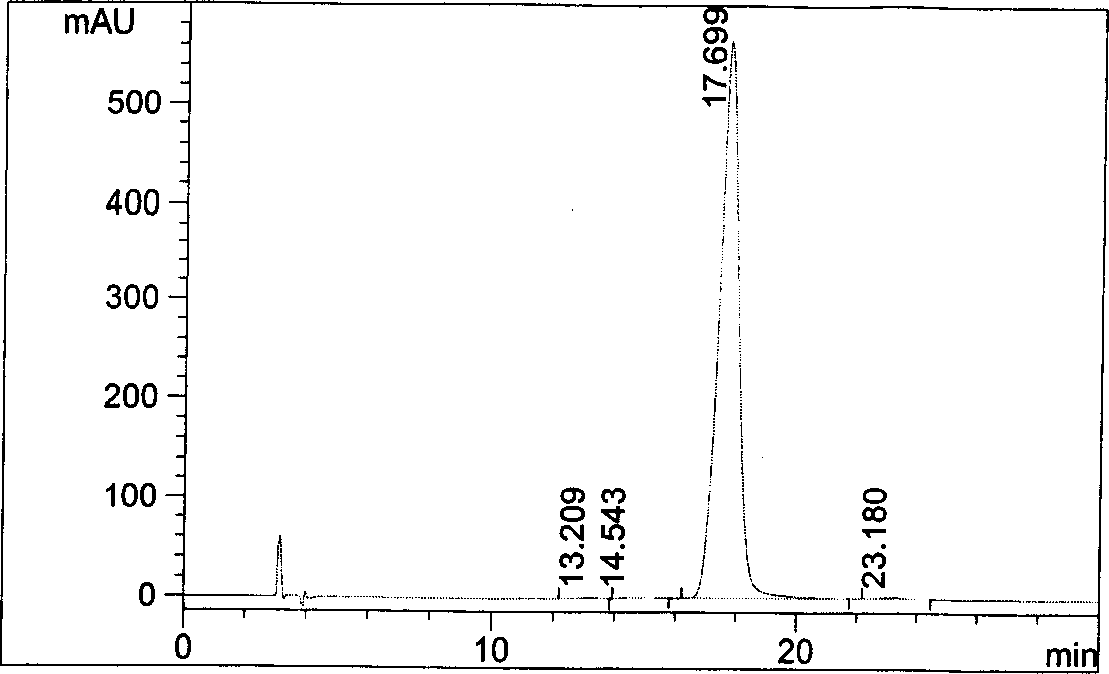
[0042] Take an appropriate amount of pitavastatin calcium tablets, approximately equivalent to 25mg of pitavastatin calcium, put it in a 50ml measuring bottle, add an appropriate amount of ethylene glycol dimethyl ether, shake to dissolve, dilute to the mark with ethylene glycol dimethyl ether, and shake well , filtered, and the filtrate was used as the test solution. Get need testing solution, carry out high performance liquid chromatography analysis according to the condition of embodiment 1, and carry out adjuvant blank test with method, the results see Figure 4 , Figure 5 .
[0043] Figure 4 Prove that the blank of excipients does not interfere with the determination, Figure 5 It shows that this method can be used for quality monitoring of preparations containing pitavastatin calcium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com