Method for separation and determination of raltitrexed and its enantiomers by high performance liquid chromatography
A technology of high performance liquid chromatography and enantiomers, which is applied in the field of pharmaceutical analysis to achieve good separation effect, accurate separation and quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
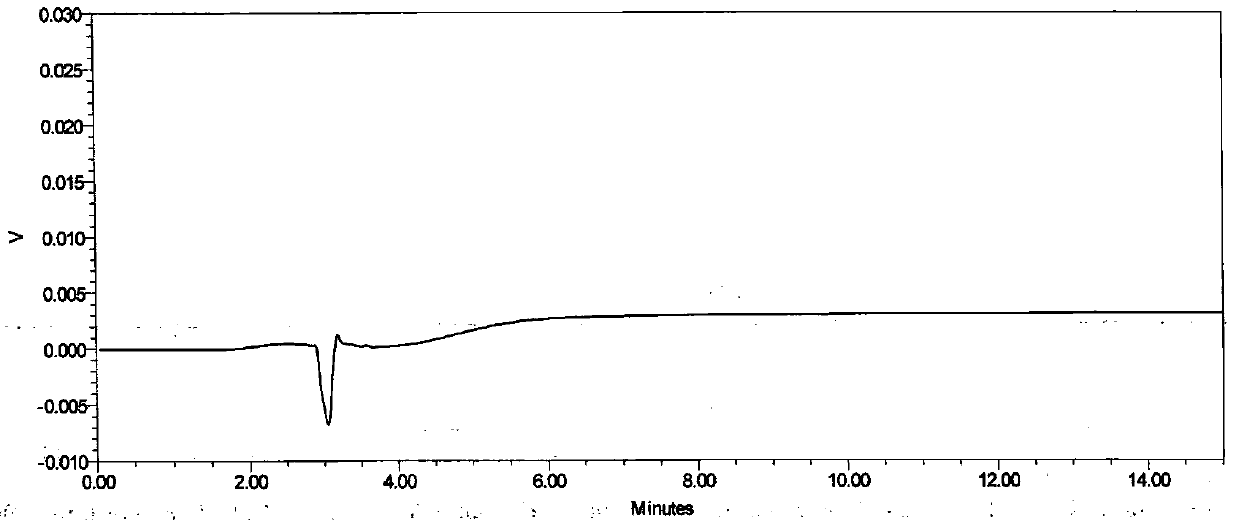
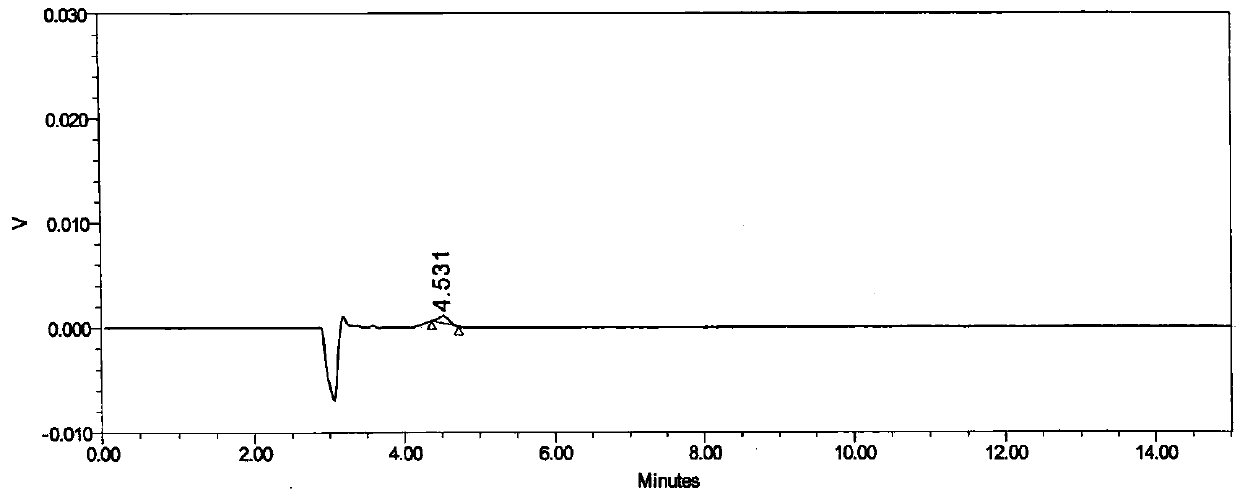
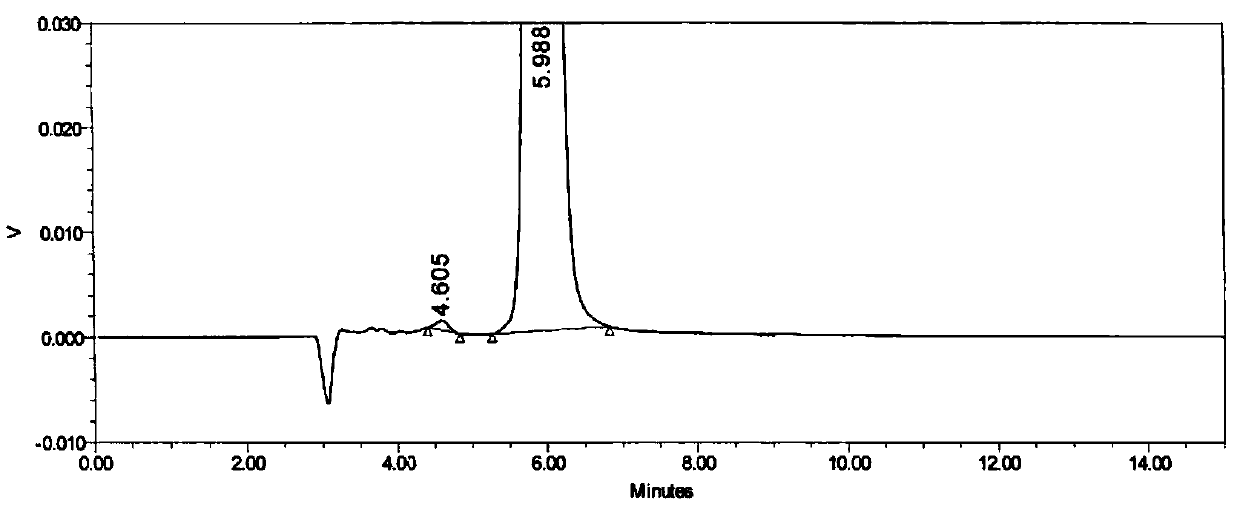
[0033] Embodiment 1 specificity test
[0034] Instrument: Shimadzu LC-20AT high performance liquid chromatography
[0035] Detector: VWD
[0036] Workstation: Empower 3 software
[0037] Chromatographic column: Daicel CHIRALPAK AD-H (4.6mm×250mm, 5μm)
[0038] Detection wavelength: 226nm
[0039] Flow rate: 1.0ml / min
[0040] Column temperature: 35°C
[0041] Injection volume: 10μL
[0042] Mobile phase: n-hexane: 0.1% trifluoroacetic acid ethanol solution (55:45)
[0043] Blank: Methanol
[0044] Raltitrexed enantiomer reference substance stock solution: Take about 12.5 mg of raltitrexed enantiomer reference substance, accurately weighed, put in a 100ml measuring bottle, add about 50ml of methanol, and dissolve it by ultrasonic, Allow to cool to room temperature, dilute to volume with methanol, shake well, and obtain. (about 125μg / mL)
[0045] Raltitrexed enantiomer localization solution: Accurately measure 1.0 mL of raltitrexed enantiomer stock solution, put it in ...
Embodiment 2
[0049] Embodiment 2 durability test
[0050] On the basis of the detection chromatographic conditions in Example 1, the conditions such as the ratio of mobile phase, column temperature, flow rate, and wavelength were changed to investigate the durability of the enantiomer detection method.
[0051] Solution preparation and assay method are with reference to embodiment 3, and each chromatographic condition is shown in the table below:
[0052]
[0053] The results are shown in the table below:
[0054]
[0055] Conclusion: Under different chromatographic conditions, the peaks of raltitrexed and the enantiomers can be separated by baseline, and the resolution meets the requirements. The method has good durability.
Embodiment 3
[0056] Embodiment 3 detection limit test
[0057] Dilute the raltitrexed isomer localization solution with 0.1mol / L sodium hydroxide aqueous solution to prepare a series of low concentration solutions, and inject samples separately until S / N≥3, which is the detection limit. Results The detection limit of enantiomer was 5.87ng / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com