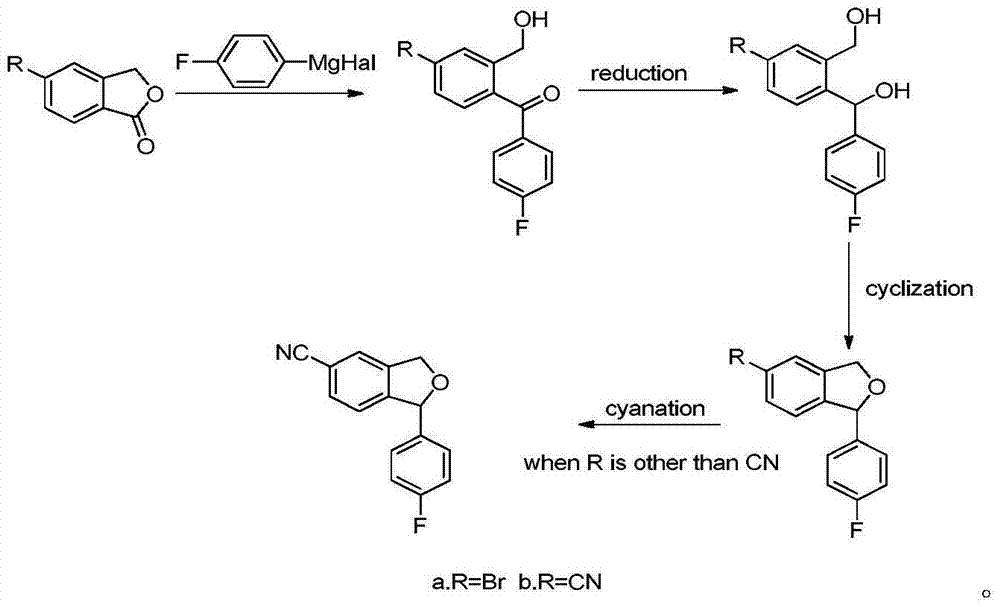
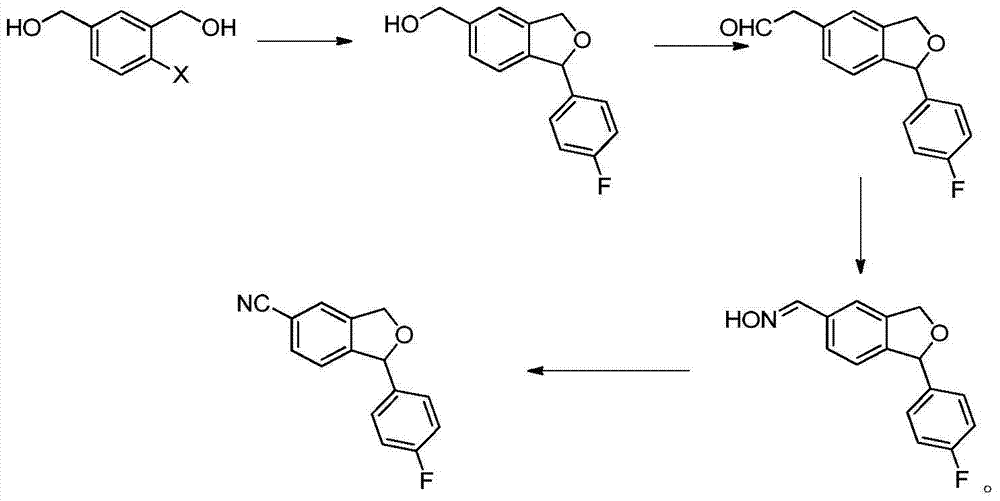
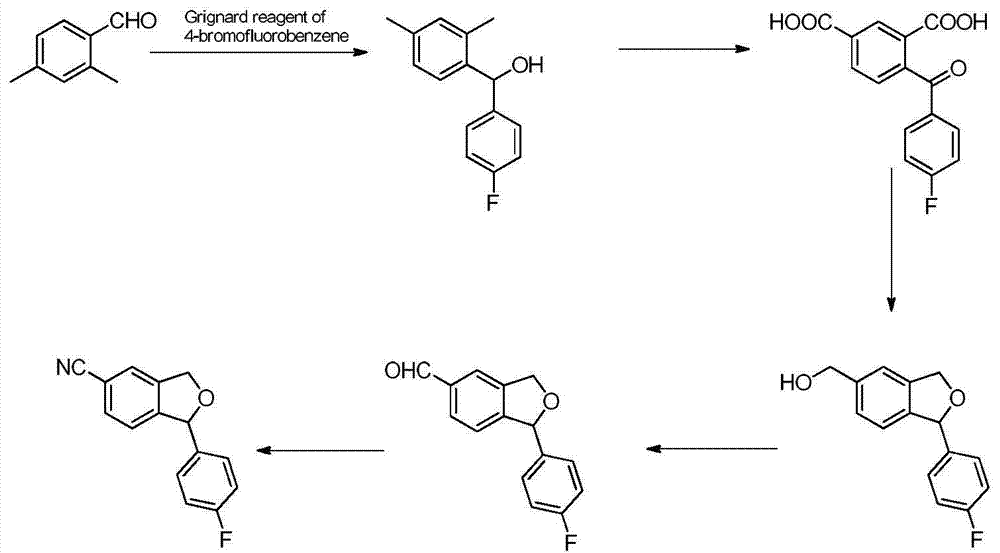
Preparation method of 5-cyanogen-1-(4-fluobenzene)-1,3-dihydrogenated-isobenzofuranone
A technology of fluorophenyl and dihydrogenation, which is applied in the direction of organic chemistry, can solve the problems of low yield, large amount of sodium borohydride, and low total yield, and achieve the effects of easy control of the reaction, simple process, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of 5-cyano-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran includes the following steps:
[0048] (1) Synthesis of p-fluorobenzoyl hydrazide
[0049] The reaction equation is:
[0050]
[0051] Ethyl p-fluorobenzoate (115 g, 683.8 mmol) was added to 1500 ml of ethanol solution, stirred until the solution was clear, 1.7 mol of hydrazine hydrate was added dropwise to the solution, and the reaction was refluxed at 90°C for 1 hour. TLC monitoring showed that after the reaction was completed, it was allowed to stand overnight, and 95 g of solid was precipitated, namely p-fluorobenzoyl hydrazide (compound 2), with a yield of 89.7%.
[0052] 1 H-NMR(300M,CDCl 3 ): 8.12 (m, 2H), 7.42 (t, 2H), 8.0 (s, 1H), 2.0 (s, 2H).
[0053] (2) Synthesis of 4-fluoro-[(5-bromo-2-hydroxyphenyl)methylene]hydrazide benzoic acid
[0054] The reaction equation is:
[0055]
[0056] 5-Bromo-2-hydroxybenzaldehyde (21g, 100mmol) was dissolved in 50ml of acetic acid aqueous solution with pH 3.5, p-f...
Embodiment 2
[0076] The preparation of 5-cyano-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran includes the following steps:
[0077] (1) Synthesis of p-fluorobenzoyl hydrazide
[0078] 0.5 mol of methyl p-fluorobenzoate was added to 1500 ml of ethanol solution, stirred until the solution was clear, 0.5 mol of hydrazine hydrate was added dropwise to the solution, and the reaction was refluxed at 100°C for 1.5 hours. According to TLC monitoring, after the reaction was completed, it was allowed to stand overnight, and a solid was precipitated to obtain 0.45 mol of p-fluorobenzoyl hydrazide with a yield of 90.0%.
[0079] (2) Synthesis of 4-fluoro-[(5-bromo-2-hydroxyphenyl)methylene]hydrazide benzoic acid
[0080] Dissolve 0.2mol of 5-bromo-2-hydroxybenzaldehyde in 50ml aqueous acetic acid with pH 3.0, add 0.2mol of p-fluorobenzoyl hydrazide, and react for 10 minutes at 40°C. After the reaction is completed, pour the reaction solution into cold water to precipitate Yellow-white solid, filtered and wash...
Embodiment 3
[0088] The preparation of 5-cyano-1-(4-fluorophenyl)-1,3-dihydro-isobenzofuran includes the following steps:
[0089] (1) Synthesis of p-fluorobenzoyl hydrazide
[0090] Add 0.5 mol of ethyl p-fluorobenzoate to 1500 ml of ethanol solution, stir until the solution is clear, add 2.5 mol of hydrazine hydrate to the solution dropwise, and react under reflux at 80°C for 1 hour. According to TLC monitoring, after the reaction was completed, it was allowed to stand overnight, and a solid was precipitated to obtain 0.46 mol of p-fluorobenzoyl hydrazide with a yield of 92.0%.
[0091] (2) Synthesis of 4-fluoro-[(5-bromo-2-hydroxyphenyl)methylene]hydrazide benzoic acid
[0092] Dissolve 0.4 mol of 5-bromo-2-hydroxybenzaldehyde in 50 ml of acetic acid with a pH of 4.0, add 0.2 mol of p-fluorobenzoyl hydrazide, and react at 30°C for 20 minutes. After the reaction is completed, pour the reaction solution into cold water to precipitate The yellow-white solid was filtered and washed with n-hexane t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com