Fluorescent probe for detecting hydrogen sulfide by virtue of fluorescence enhancement as well as synthetic method and application of fluorescent probe
A fluorescence-enhanced and fluorescent molecular probe technology is applied in the preparation of fluorescence-enhanced detection of hydrogen sulfide fluorescent probes, and the application field of hydrogen sulfide detection, and can solve the problems of large background interference, slow hydrogen sulfide response speed, and high detection limit. Achieve the effect of high sensitivity, wide detection range and fast response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of compound 3
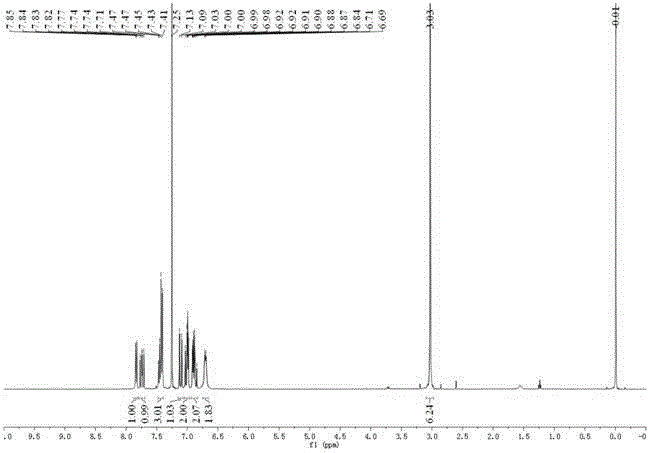
[0034] In a 100 mL single-necked flask, add 4-dimethylaminocinnamaldehyde (0.35 g, 2 mmol), o-hydroxyacetophenone (0.27 g. 2 mmol) and sodium hydroxide (0.2 g, 5 mmol) dissolved in 20 ml In methanol, the temperature was raised to reflux for 5 hours. After the reaction was completed, cool to room temperature, pour the reaction solution into ice water, adjust the pH value to about 2 with hydrochloric acid, and precipitate a large amount of solids. The solid was collected by suction filtration, dried, and recrystallized from ethanol. The solid product 0.38 g (yield: 65 %) was obtained as compound 2.
[0035] δ H (400 MHz, CDCl 3 ): δ 7.83 (dd, J = 8.1, 1.3 Hz, 1H), 7.74 (dd, J = 14.5, 11.2 Hz, 1H), 7.48 – 7.39 (m, 3H), 7.11 (d, J = 14.5 Hz, 1H), 7.04 – 6.97 (m, 2H), 6.94 – 6.83 (m, 2H), 6.70 (d, J = 7.7 Hz, 2H), 3.03 (s, 6H).
[0036]
Embodiment 2
[0037] Embodiment 2: the preparation of compound 2
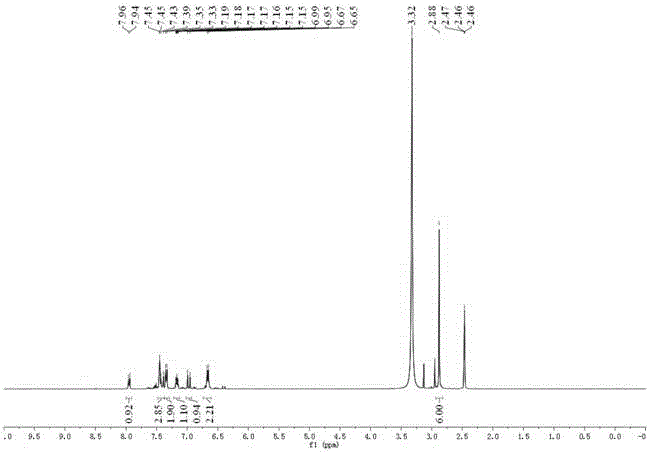
[0038] Under ice cooling, compound 3 (293 mg, 1 mmol) and 2 mL of 20% sodium hydroxide solution were dissolved in 20 mL of methanol solution, and 1 mL of 30% hydrogen peroxide was added dropwise to the reaction solution. After the dropwise addition, the temperature was raised to reflux for 3 hours. After the reaction was completed, the temperature was lowered to room temperature, and a precipitate was precipitated. Collect the precipitate and dry the obtained product as compound 2. 175 mg (yield: 57 %)
[0039] 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.95 (d, J = 8.4 Hz, 1H), 7.43 (dd, J = 17.0, 9.1 Hz, 3H), 7.34 (d, J = 8.8 Hz, 2H), 7.17 (ddd, J = 7.9, 5.0, 2.9 Hz, 1H), 6.97 (d, J = 16.2 Hz, 1H), 6.66 (d, J = 8.8 Hz, 2H), 2.88 (s, 6H).
[0040]
Embodiment 3
[0041] Embodiment 3: the preparation of fluorescent probe
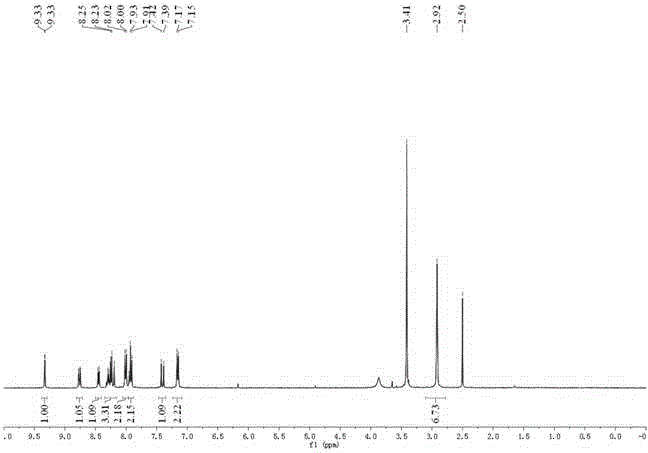
[0042] Compound 2 (31 mg, 0.1 mmol), 2,4-dinitrofluorobenzene (22 mg, 0.12 mmol) and potassium carbonate (17 mg, 0.12 mmol) were dissolved in 10 ml of acetonitrile and refluxed for 8 hours. Cool down, pour into water, extract with dichloromethane, dry over anhydrous sodium sulfate, and separate by column chromatography (dichloromethane) to obtain 36 mg of the product (yield: 76 %), which is the probe compound.
[0043] 1 H NMR (400 MHz, DMSO- d 6 ) δ 9.33 (d, J = 2.7 Hz, 1H), 8.76 (dd, J = 9.3, 2.8 Hz, 1H), 8.45 (d, J = 7.9 Hz, 1H), 8.35 – 8.16 (m, 3H), 8.01 (d, J = 8.8 Hz, 2H), 7.93 (t, J = 8.8 Hz, 2H), 7.41 (d, J = 15.9 Hz, 1H), 7.16 (d, J = 8.8 Hz, 2H), 2.92 (s, 6H).
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com