Sensitive fluorescent lighting probe method for determining CMC (critical micelle concentration) of surfactant
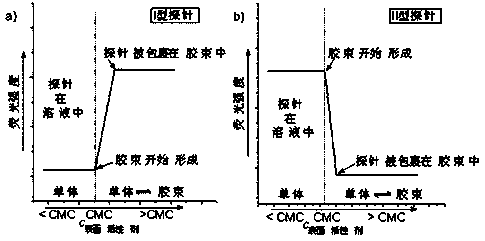
A critical micelle concentration and surfactant technology, applied in luminescent materials, chemical instruments and methods, chemiluminescence/bioluminescence, etc., can solve problems affecting micelle formation, failing to find type II probes, reducing TPE sensitivity, etc. , to achieve the effects of eliminating errors, high sensitivity, and simplifying operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
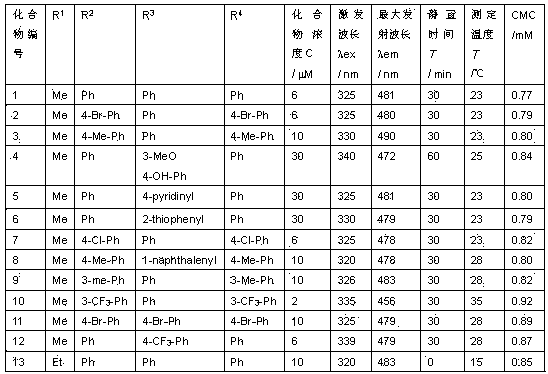
[0057] S1. Dimthyl 1,2,3,6-tetrahydro-1,2,3 -triphenylpyrimidine-4,5-dicarboxylate, compound 1) was prepared as a stock solution at a concentration of 1 mM in ethanol.
[0058]
[0059] Compound 1
[0060] S2. In a 100mL volumetric flask, add 288 mg sodium dodecyl sulfate (SDS), add double distilled water until the sample is dissolved, then add 0.6 mL of the stock solution prepared by S1., and then use double distilled water to determine In the constant solution, the concentration of SDS is 10 mM, and the concentration of compound 1 is 6 μM in the constant solution.
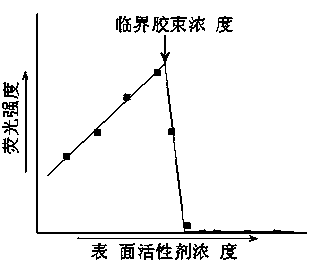
[0061]S3. Prepare the non-fluorochrome solution prepared in S2. to prepare SDS sample solutions with different concentrations for the determination of fluorescence and SDS critical micelle concentration (CMC). Specific steps are as follows:
[0062] At 25°C, according to Table 1, take different volumes of the fixed solution prepared in step S2. into eleven 10 mL volumetric flasks, add double distilled water...
Embodiment 2
[0069] S1. Dimethyl 1,3-bis(4-bromophenyl)-1,2,3,6-tetrahydropyrimidine-4,5-dicarboxylate (dimethyl 1,3-bis(4 -bromophenyl)-1,2,3,6-tetrahydro-2-phenylpyrimidine-4,5-dicarboxylate, compound 2) was prepared as a stock solution with a concentration of 1 mM in ethanol.
[0070]
[0071] Compound 2
[0072] S2. In a 100mL volumetric flask, add 576 mg sodium dodecyl sulfate (SDS), add double distilled water until the sample is dissolved, then add 1.2 mL of the stock solution prepared by S1., and then use double distilled water to determine In order to obtain a non-fluorescence fixed solution, the concentration of SDS in the fixed solution is 20 mM, and the concentration of compound 2 is 12 μM.
[0073] S3. Prepare the non-fluorochrome solution prepared in S2. to prepare SDS sample solutions with different concentrations for the determination of fluorescence and SDS critical micelle concentration (CMC). Specific steps are as follows:
[0074] At 27°C, according to Table 2, tak...
Embodiment 3
[0080] S1. Dimethyl 1,2,3,6-tetrahydro-2 -phenyl-1,3-dip-tolylpyrimidine-4,5-dicarboxylate, compound 3) was prepared as a stock solution with a concentration of 1 mM in THF.
[0081]
[0082] Compound 3
[0083] S2. In a 100mL volumetric flask, add 72.8 mg cetyl trimethyl ammonium bromide (CTAB), add double distilled water until the sample is dissolved, then add 1 mL of the stock solution prepared by S1., then Double-distilled water was used for constant volume, so that a non-fluorescent constant solution was obtained. In the constant solution, the concentration of CTAB was 2 mM, and the concentration of compound 3 was 10 μM.
[0084] S3. Use the non-fluorescent solution prepared in S2. to prepare different concentrations of CTAB sample solutions for the determination of fluorescence and CTAB critical micelle concentration (CMC). Specific steps are as follows:
[0085] According to Table 3, take different volumes of the fixed solution prepared according to step S2. into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Critical micelle concentration | aaaaa | aaaaa |
| Critical micelle concentration | aaaaa | aaaaa |
| Critical micelle concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com