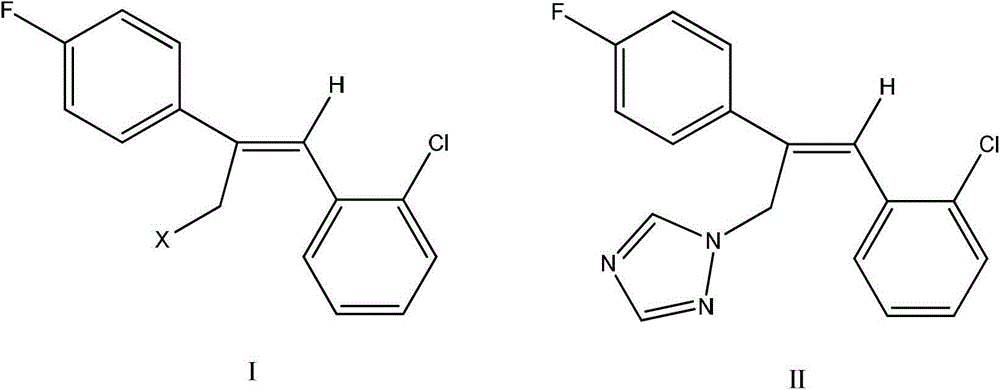
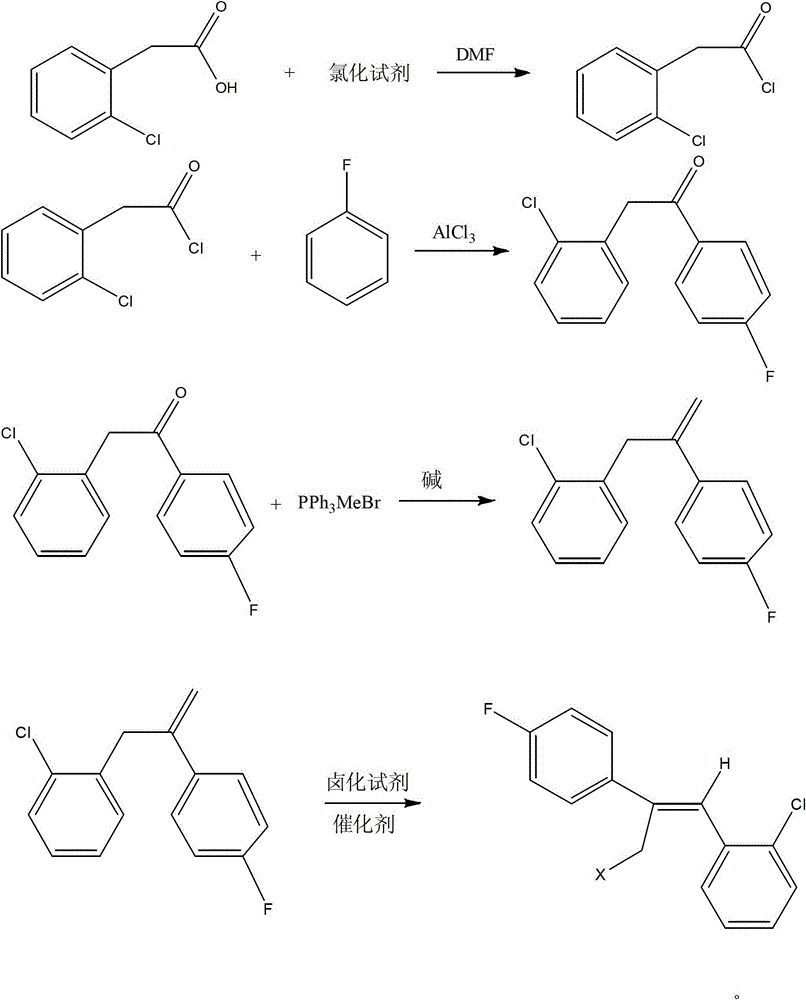
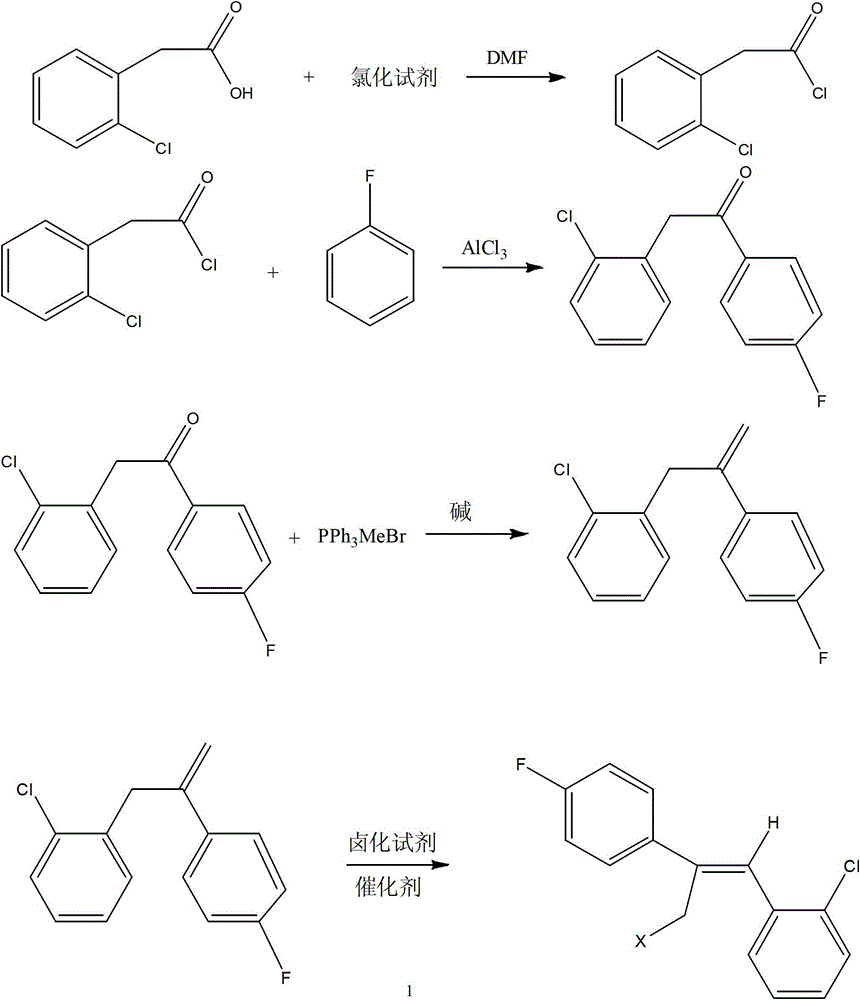
Preparation method of epoxiconazole intermediate (Z)-2-(4-fluorophenyl)-1-(2-chlorphenyl)-3-halogen propylene
A technology of halogenated propylene and chlorophenyl, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of high cost and low yield, achieve less side reactions, high product yield, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] The technical solutions of the present invention will be further described below in conjunction with specific examples, but the present invention is not limited to these examples. In these examples, unless otherwise stated, all reagents used were of reagent grade and measured in grams (g).
[0017] (1) Synthesis of o-chlorophenylacetyl chloride - thionyl chloride method
[0018] Add 17.0g (0.1mol) of o-chlorophenylacetic acid into the reaction flask, add 500mL of dichloromethane and stir to dissolve, then add 0.2g of DMF as a catalyst, raise the temperature to 35°C, add 17.8g (0.15mol) of thionyl chloride dropwise To the above solution, the dropwise addition was completed in one hour, then the temperature was raised to 45° C., and the reaction was terminated after reflux reaction for 3 hours. The solvent and excess thionyl chloride were distilled off to obtain 18.5 g (0.098 mol) of o-chlorophenylacetyl chloride, with a yield of 98%.
[0019] (2) Synthesis of o-chlorop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com