Patents
Literature
55 results about "Correcting agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
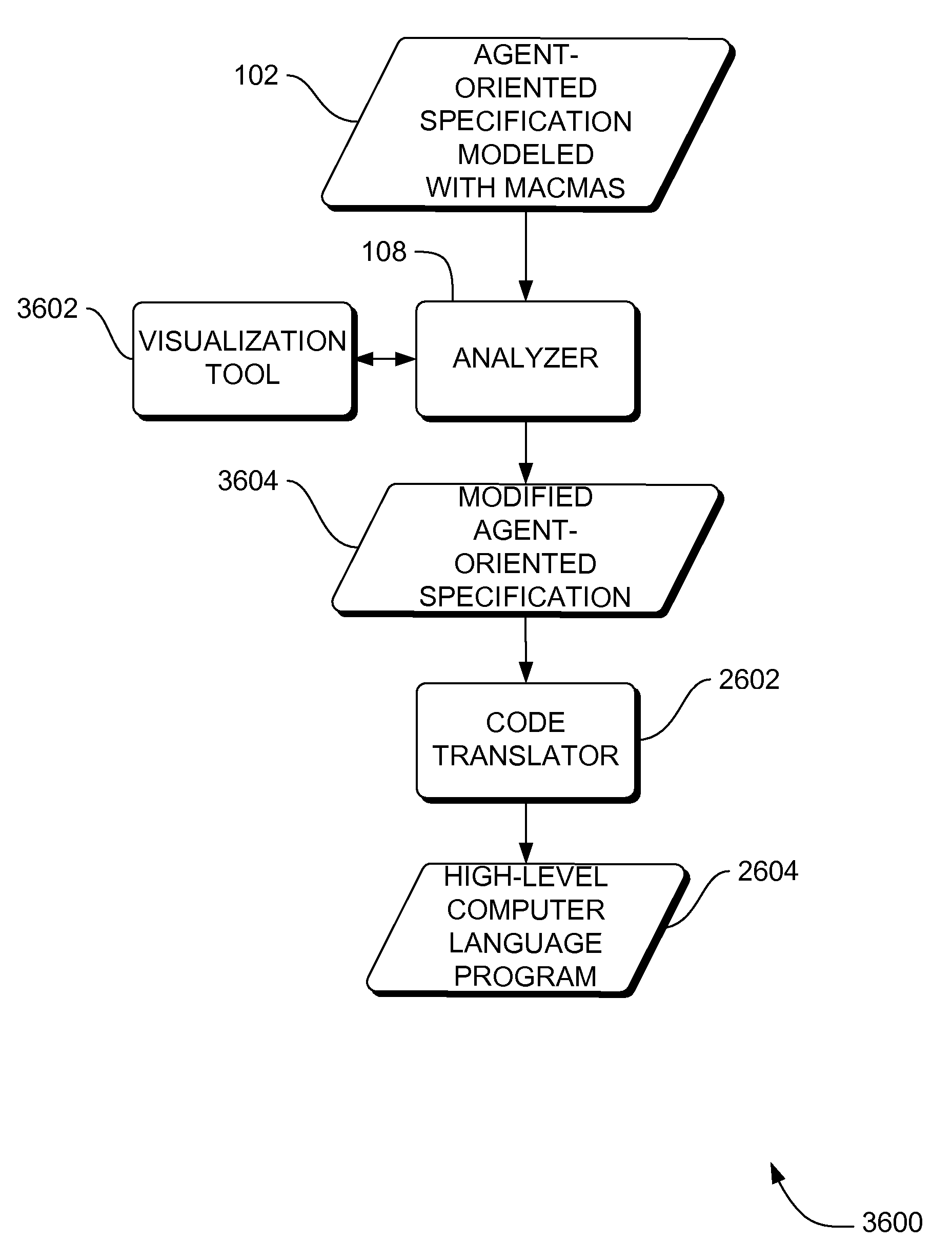
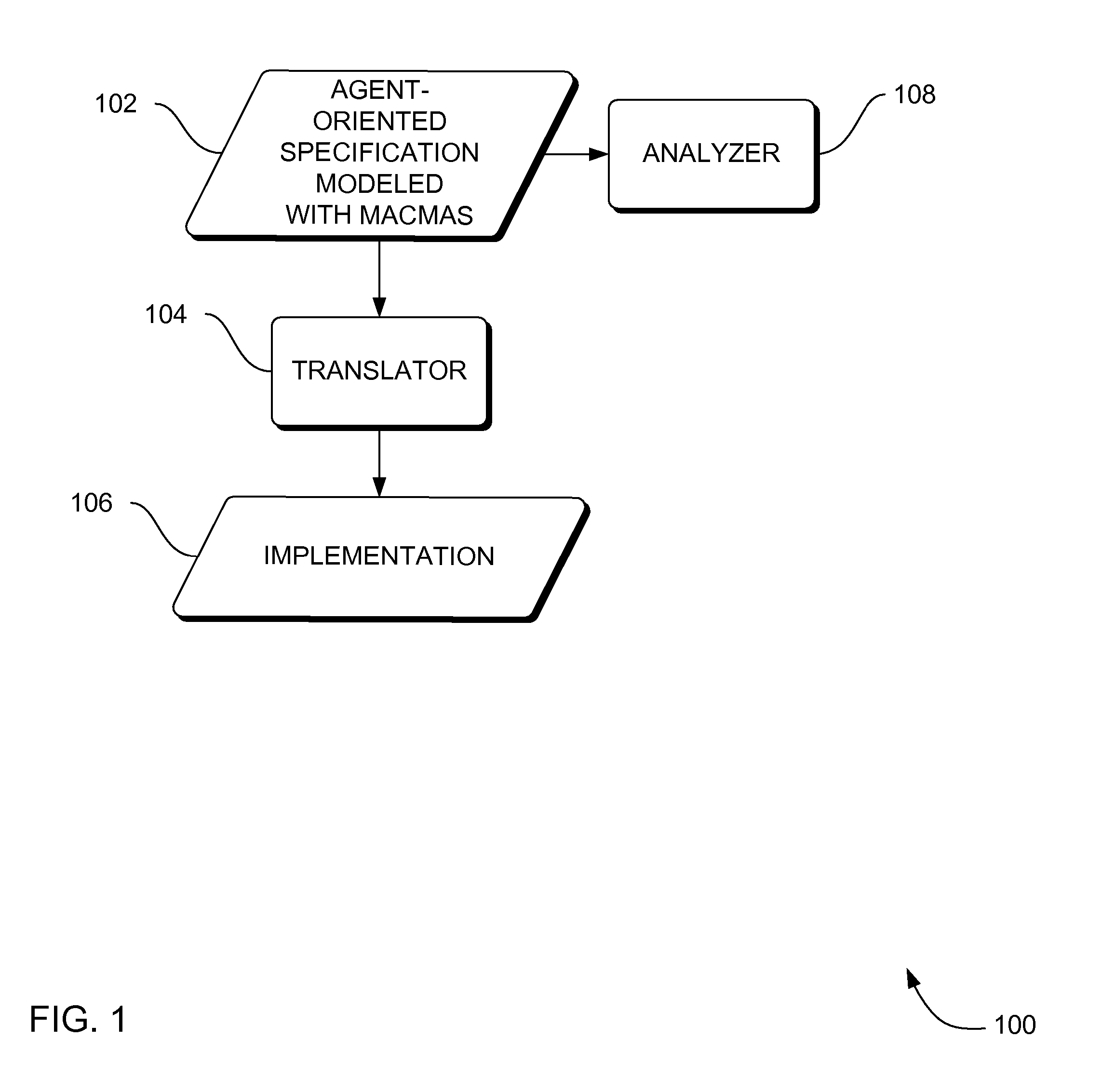
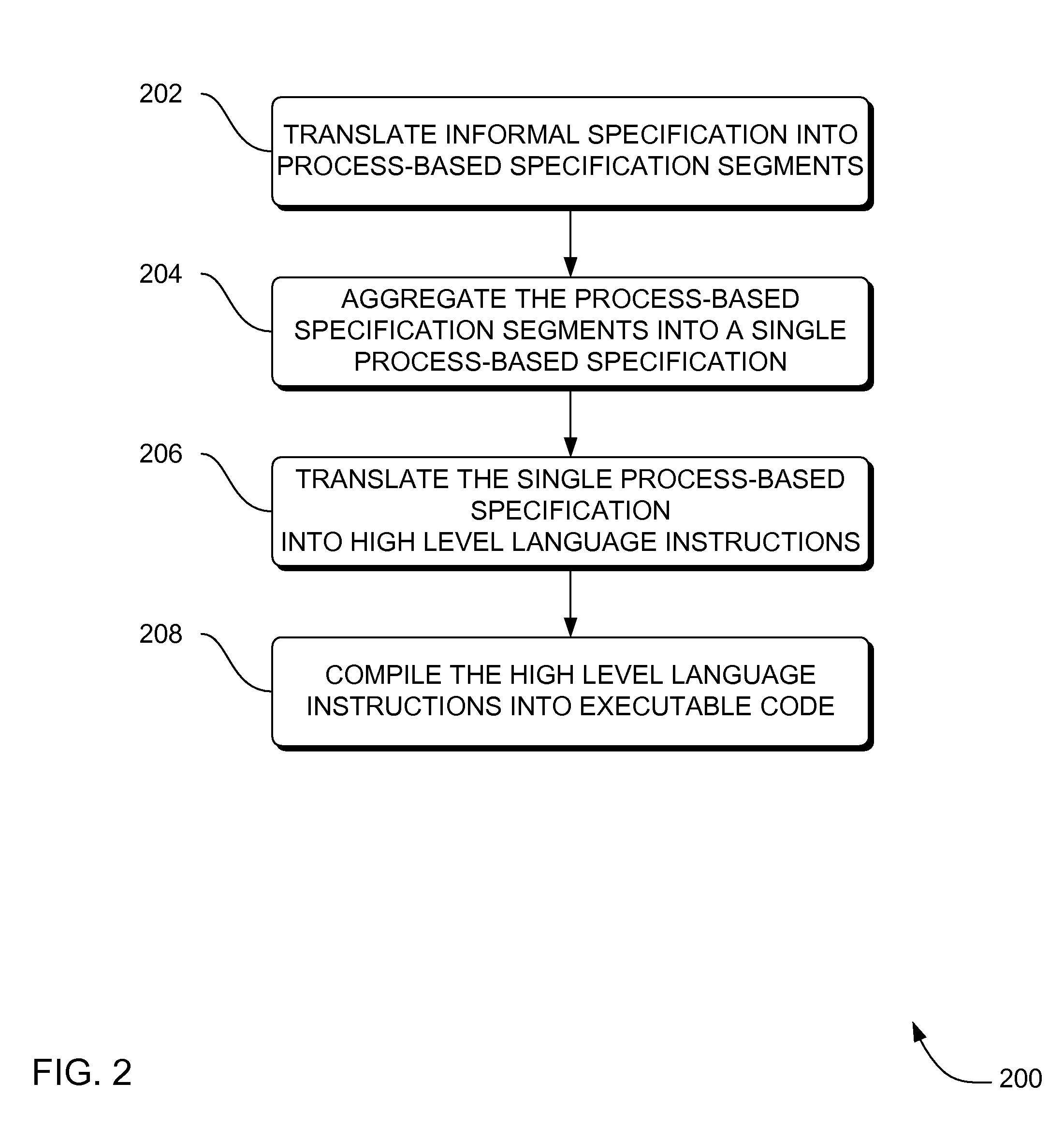
Systems, methods and apparatus for modeling, specifying and deploying policies in autonomous and autonomic systems using agent-oriented software engineering
ActiveUS7992134B2Improve policyPromote resultsRequirement analysisSpecific program execution arrangementsProgramming languageAgent-oriented software engineering
Systems, methods and apparatus are provided through which in some embodiments, an agent-oriented specification modeled with MaCMAS, is analyzed, flaws in the agent-oriented specification modeled with MaCMAS are corrected, and an implementation is derived from the corrected agent-oriented specification. Described herein are systems, method and apparatus that produce fully (mathematically) tractable development of agent-oriented specification(s) modeled with methodology fragment for analyzing complex multiagent systems (MaCMAS) and policies for autonomic systems from requirements through to code generation. The systems, method and apparatus described herein are illustrated through an example showing how user formulated policies can be translated into a formal mode which can then be converted to code. The requirements-based programming systems, method and apparatus described herein may provide faster, higher quality development and maintenance of autonomic systems based on user formulation of policies.
Owner:NASA
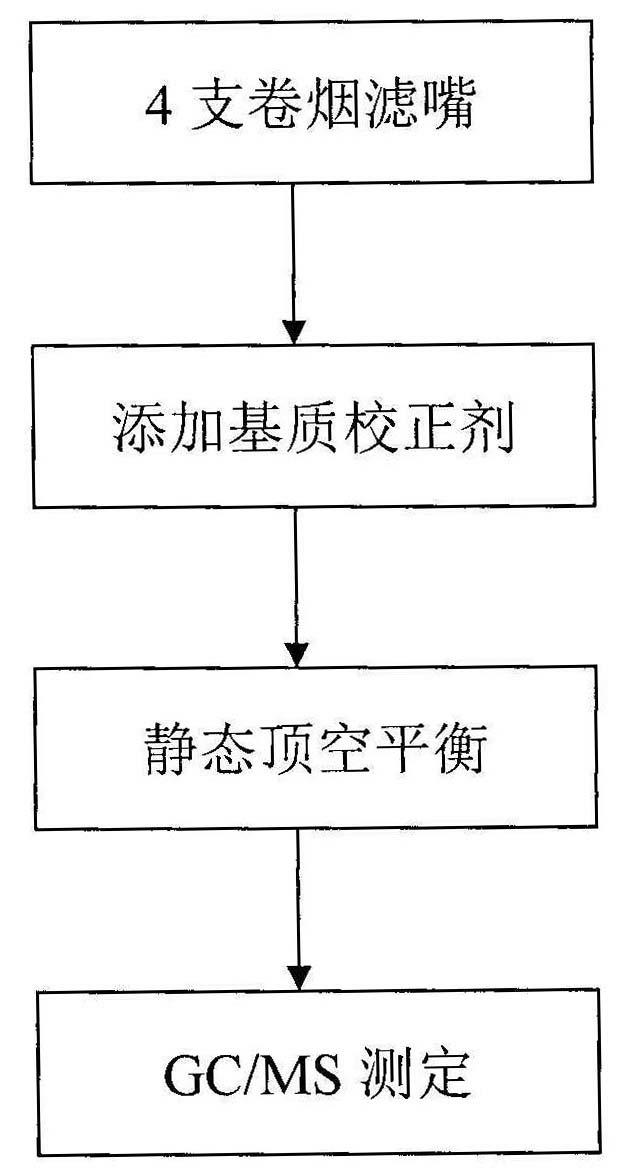
Method for detecting volatile organic compound in cigarette filter
InactiveCN102128906AEasy to handleEasy processing conditionsComponent separationInterference factorMass spectrography
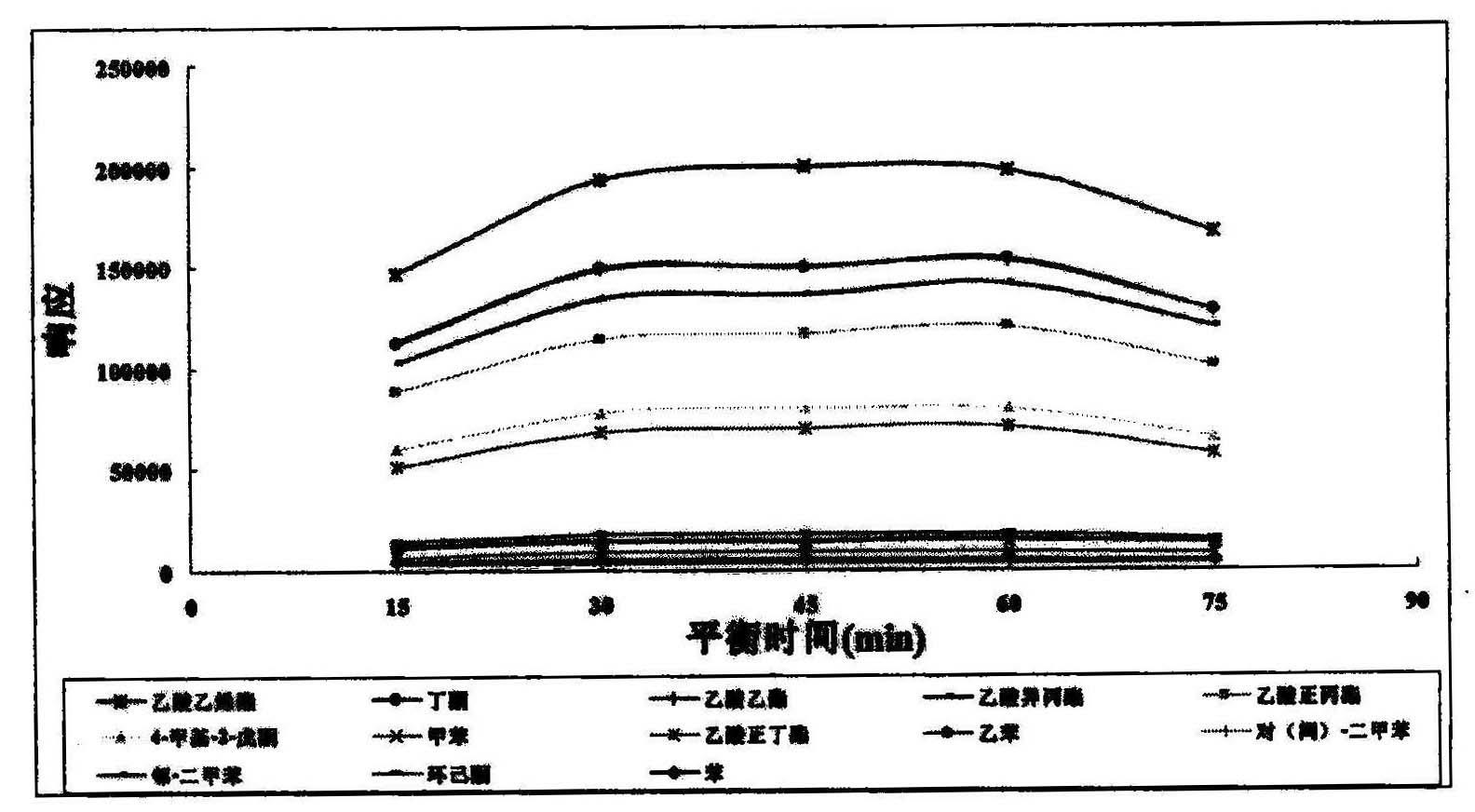
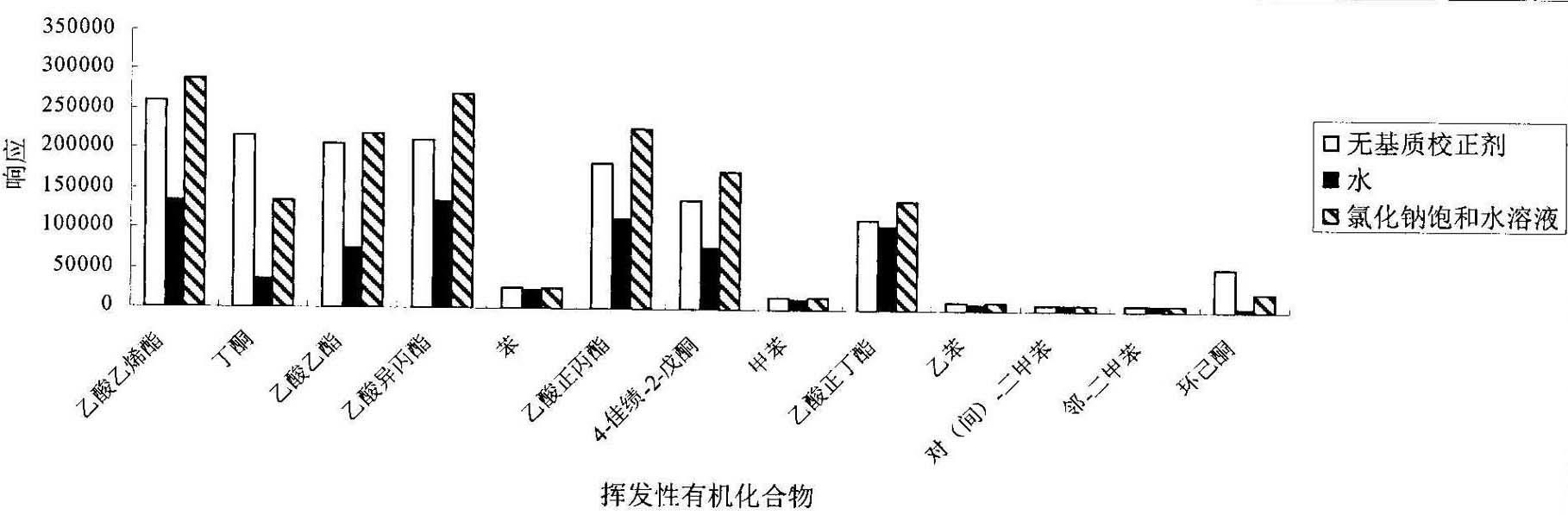
The invention provides a method for detecting volatile organic compound in a cigarette filter, which comprises the following steps: a) preparing a sample; b) preparing a matrix correcting agent; c) preparing an interior label solution; d) preparing a standard sample; e) preparing a standard working solution; f) performing detection by using headspace-gas chromatography / mass-spectrography; and g) calculating a detection result of the volatile organic compound in the sample. The invention establishes the method for detecting the volatile organic compound residue in the cigarette filter for the first time. By using the saturation sodium chloride aqueous solution as the matrix correcting agent and the fluorobenzene as the interior label, the matrix effect of a solid absorption matrix (namely the cigarette filter) is efficiently reduced and the error caused by the poor repeatability of the pre-treatment and the low precision of instrument is reduced. In the whole process, no organic solvent is used. The method has the advantages of environmental friendliness, energy-saving and low consumption. The detecting method has few interference factors and good repeatability, is accurate in measuring result, is simple to operate, and can be used for obviously increasing the sensitivity of headspace analysis.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Method of preparing smectite suspension agent
InactiveCN101129326AFully dispersedDisperse fastDigestive systemPharmaceutical delivery mechanismMontmorilloniteColloid
The invention provides a montmorillonite mixing-floating agent for treating loose bowel movement and making method, which comprises the following steps: (1) drawing formulating amount montmorillonite, correcting agent, preservative agent, anddeionized water; (2) adding the montmorillonite into the anddeionized watert to stir uniformly to add the correcting agent and the preservative agent to stir uniformly; (3) producing the mixing liquid to form the uniform mixing-floating agent with grain fineness of the montmorillonite less than10mu m through colloidal mill that can be placed with homogenizer or ultrasonic machine; (4) sterilizing to split charging to get the mixing-floating agent. The invention has merits of fitting viscosity for splitting dose accurately and reducing production cost without assisting-floating agent, which makes the montmorillonite disperse thoroughly through the colloidal mill with finer grains that can be distributed in body's digestive tract rapidly after taking and with better effect.
Owner:JINAN KANGZHONG PHARMA TECH DEV
Traditional Chinese medicine preparation for treating insomnia and preparation method and use thereof
InactiveCN101450122ALarge prescription volumeMore medicinalNervous disorderPill deliveryAlcoholAdemetionine
The invention discloses a traditional Chinese medicine preparation for treating insomnia and preparation method thereof. The preparation is made from (stir-fried) spine date seed and schisandra according to a certain weight proportion by the process of: alcohol extracting the decoction pieces, decompressing and recovering to tastelessness alcohol, concentrating to obtain alcohol extracted concentrated solution and herb residue; decocting the herb residue by water, concentrating the decoction solution and centrifuging; re-concentrating the supernatant, combining with the alcohol extracted concentrated solution, concentrating, drying to extract powder; and adding proper amount of auxiliary material or taste correcting agent and making into granules, capsules tablets or pills. The pharmacological tests shows that: the preparation in the invention has good therapeutic action to insomnia, and can tranquilize the mind.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Ebstine solid oral preparation and its preparing method
ActiveCN101161233AImprove solubilityIncrease dissolution ratePill deliveryCapsule deliveryOrganic acidOrally disintegrating tablet
An ebastine solid oral preparation and preparation method are disclosed in the present invention. Made of ebastine, ethanol solution, cosolvent, diluent or filler, disintegrating agent, taste correcting agent, glidants or dispersing agent, effervescing agent, lubricant in proportion, its step is that firstly adding ebastine into ethanol solution, and then adding cosolvent organic acid, leading it to dissolve entirely; secondly mixing the diluent or filler, disintegrating agent, taste correcting agent, effervescing agent with dispersing agent, to obtain a mixer; third mixing the front two, preparing soft material, preparing granules, then adding disintegrating agent, effervescing agent, taste correcting agent, glidants and lubricant, tabletting or filling capsules, packing to obtain a solid oral preparation. The present invention preparing oral disintegrable tablet, dispersing tablet have short disintegrating time, good dispersing state. The obtained oral disintegrable tablet, dispersing tablet and chewing tablet have good taste and mouth feel, the obtained solid oral preparations dissolving out are all rapid. The method is easy, simple operation, without needing special equipment, and lower production cost.
Owner:HUBEI LIYI PHARM TECH CO LTD
Production method of porous calcium carbonate
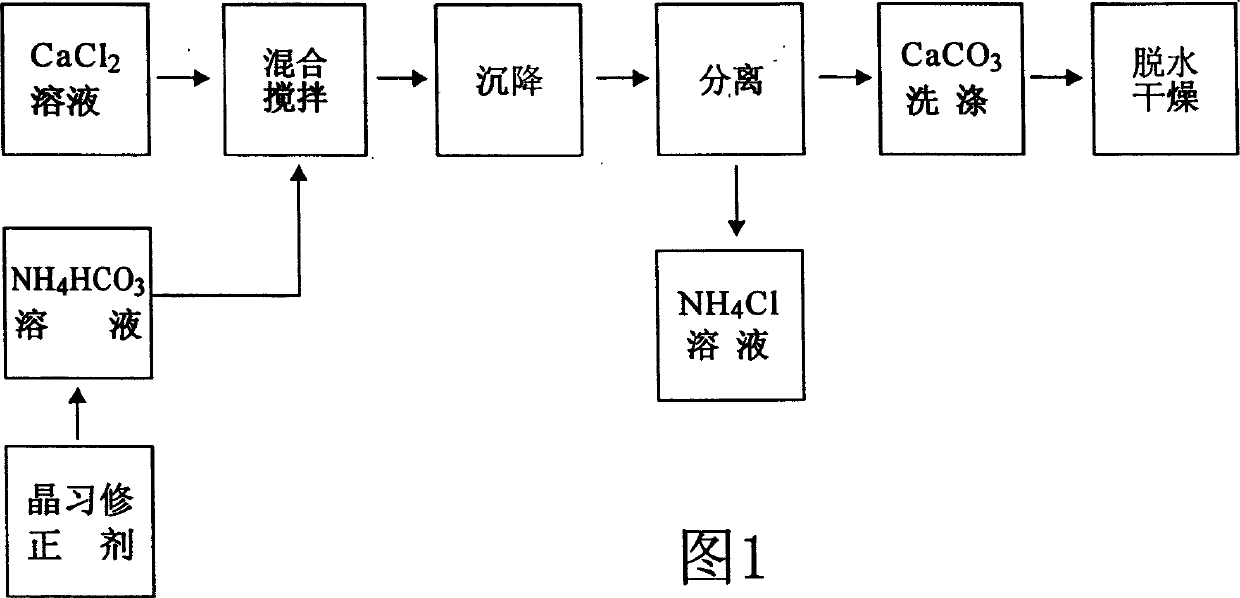
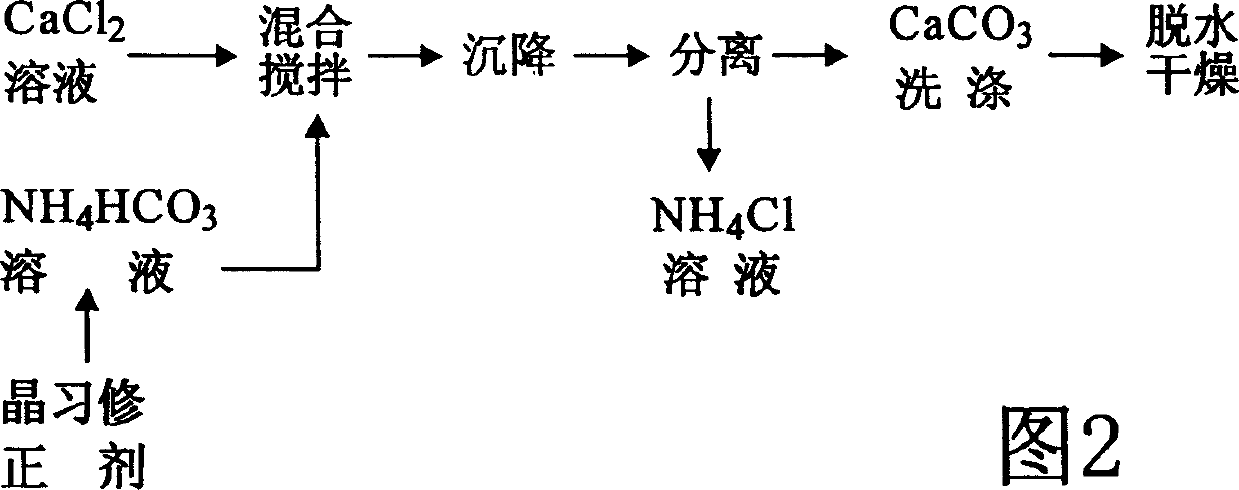
InactiveCN1515495ALow densityImprove adsorption capacityCalcium/strontium/barium carbonatesChemical reactionAmmonium Hydrogen Carbonate
The preparation method of porous calcium carbonate includes the folloiwng steps: preparing calcium chloride aqueous solution and ammonium hydrogen carbonate aqueous solution, adding crystal habit correcting agent whose adding quantity is 0.01-0.05% of total mass of the above-mentioned two solutions; mixing them at 25-30 deg.C, fully stirring at normal pressure until the air is reacted, settling reaction product for 0.5-1 hr., separating sediment calcium carbonate from ammonium hydride solution, then washing said sediment calcium carbonate by using water, dewatering, drying so as to obtain the porous calcium carbonate product with strong adsorption force and penetrating quality. It can be extensively used in the industries of plastics paper-making, paint, printing ink and domestic chemicals, etc.
Owner:谢作文
Medicinal composition for treating anemopyretic cold and its preparation method
The invention discloses a medicine compound for curing wind-heat type cold and its manufacturing method. The medicine compound is made up of honeysuckle, root of large-flowered skullcap, bupleurum root, southernwood, weeping forsythia, giant knotweed, gardenia, root-bark of peony, notopterygium, liquorice according a certain proportion. The manufacturing method is: pupleurum root, notopterygium, honeysuckle and southernwood are distilled with water; the mother liquid and volatile oil are for backup. The root-bark of peony is distilled with water and acquires the cortex moutan phenol crystal; or packages the crystal for backup. All the medicine dredges are combined, and they are stewed with water, the filtered liquid and the distilled water liquid are combined for backup. The root of large-flowered skullcap, giant knotweed, gardenia and liquorice are stewed in water, the filtered liquid is combined with above mentioned medicine liquid, and they are condensied to thick extractive plaster. Finally, the excepient and flavor correcting agent into the medicine, thus the product can be acquired. The medicine has functions of surface penetrating and heat clearing and detoxification.
Owner:扬州中惠制药有限公司
Astragalus essence effervescent tablets and preparation method thereof
InactiveCN101181340ADisintegrates quicklyReduced disintegrationDigestive systemPill deliveryFlavorEffervescent tablet
The invention relates to a radix astragali essence effervescent tablet containing the radix astragali and a preparing method thereof. The radix astragali essence effervescent tablet is made by applying the extract powder which is made by the Chinese traditional medical radix astragali as raw material, adding effervescent agent, disintegrating agent, filling agent, flavor correcting agent and lubricant to conduct a wet method, making grains and tabletting. The invention is characterized by convenience, rapidness and novelty and is unlimited by water temperature when in use, The effervescent tablet contains no cane sugar and has sweet taste, thus improving the adaptability of the patient and assuring the curative effect. The invention adopts the technique of combining the effervescent agent and the disintegrating agent to prepare the effervescent tablet, improves the disintegration of the effervescent tablet, reduces the use of the effervescent agent and lowers the cost. Moreover, the invention has simple technique and strong controllability and facilitates the quality control of the products.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD
Lightweight high-strength porous ceramsite and preparation method thereof
InactiveCN109592989AImprove performanceBulk density adjustableCeramic materials productionCeramicwareFoaming agentSludge
The invention belongs to the field of solid waste treatment and resource utilization, and particularly relates to a lightweight high-strength porous ceramsite and a preparation method thereof. The preparation method of the lightweight high-strength porous ceramsite comprises the following steps of: respectively drying, pulverizing and sieving sludge, steel slag, a correcting agent and a foaming agent which are used as raw materials, then carrying out ball-milling, uniformly mixing, granulating, drying, pre-firing, sintering and quickly cooling to obtain the lightweight high-strength porous ceramsite. The lightweight high-strength porous ceramsite comprises the following raw materials in parts by mass: 50-70 parts of sludge, 10-25 parts of steel slag, 10-30 parts of collecting agent, and 0-10 parts of foaming agent. The lightweight high-strength porous ceramsite is prepared by compounding the sludge and the steel slag for the first time, adjusting the ratio of the raw materials and designing the calcination process. The prepared lightweight porous high-strength porous ceramsite has excellent performance, and the bulk density, strength and water absorption rate of the lightweight porous high-strength porous ceramsite are adjustable. The preparation method has simple process and solves the problems of difficult disposal of the sludge and the steel slag, low utilization rate and added economic value and the like; and compared with the traditional disposal method, the preparation method greatly improves the added economic value.
Owner:WUHAN UNIV OF TECH
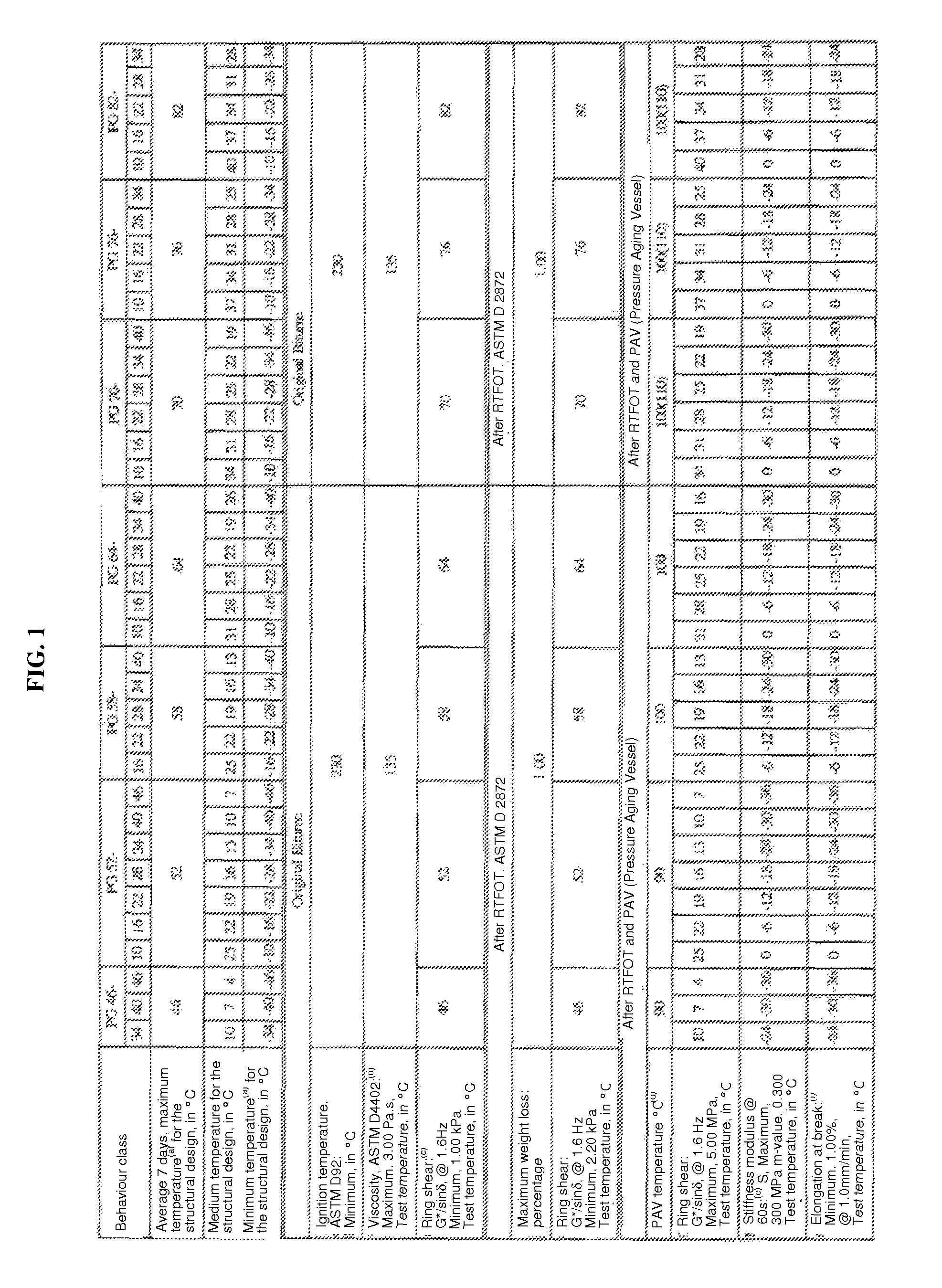
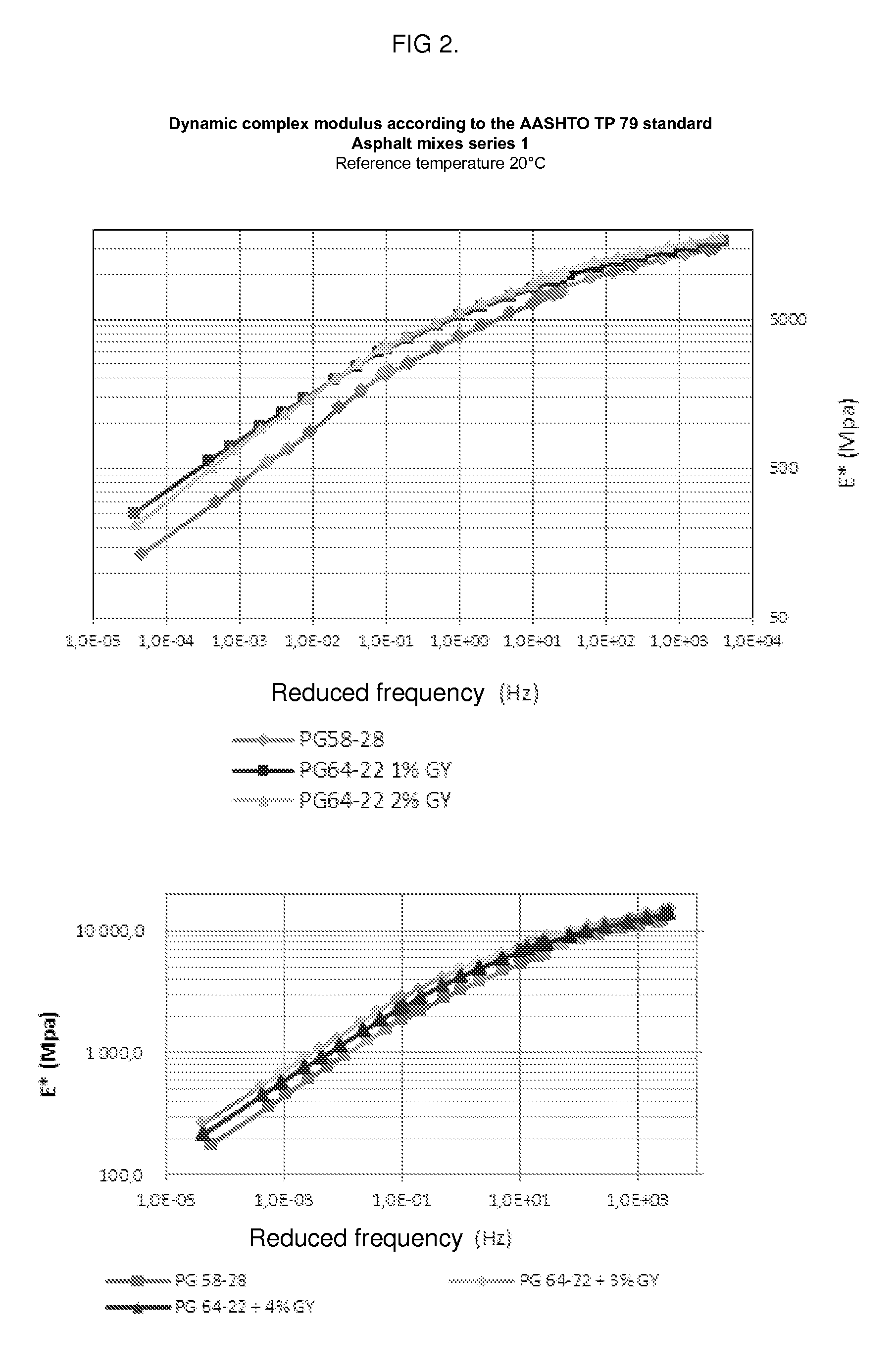
Binder modified with glycerol for making asphalt mixes with a hicontent of recycled bituminous materials
InactiveUS20140130712A1Simple methodLow costSolid waste managementBituminous material adhesivesBituminous materialsGlycerol
Owner:COLAS LTD
Arginine ibuprofen oral disintegrating tablets and preparation method thereof
InactiveCN101455653AGreat tasteHigh hardnessOrganic active ingredientsAntipyreticFiller ExcipientOrally disintegrating tablet
The present invention relates to an orally disintegrating tablet of febrifuge and analgesic ibuprofen arginine and a preparing method thereof. The orally disintegrating of ibuprofen arginine according to the invention is composed of active component of ibuprofen arginine and appropriate medical supplementary material. The weight percent is as follows: 20%-60% of ibuprofen arginine, and 40%-80% of supplementary material. The supplementary material comprises the following components: one component or a plurality of components selected from thinning agent (filling agent), disintegrating agent, adhesive, wetting agent, lubricant, flavor correcting agent, colorant and effervescence disintegrating agent. The orally disintegrating of ibuprofen arginine can adopt the flavor correcting agent, mucilage, packing technique or integratedly use the plurality of methods for covering the biting taste of principal agent. The orally disintegrating of ibuprofen arginine of the invention has the advantages of excellent mouth feel, no requirement of water in medicine taking, quick disintegrating in oral cavity, quickly medicine releasing in stomach, guarantee of quick function, better hardness, no requirement of special device in production process and suitability of industrial production.
Owner:TIANJIN MEDICAL UNIV
Donky-hide blood-supplementing tonic and its preparation method
InactiveCN1579435ASignificant effectGood red blood cellsUnknown materialsBlood disorderSide effectMedicine
The invention discloses an ass-hide glue blood enriching oral liquid and its manufacturing method which relates to a blood enriching medicine with Chinese traditional medicine as materials and its manufacturing method. The ass-hide blood enriching oral liquid uses ass-hide glue, prepared rehmannia root, co donopsis pilosula root, astragalus, medlar, lagehead atractylodes, taste correcting agent, aseptic as materials, and the oral liquid can be produced with current processing technology, the effect is accurate through several years of clinic experiences, the blood enriching effect is prominent, and it has no toxin and side effects.
Owner:FUJIAO GROUP CORP SHANDONG
Orally disintegrating tablet of gypenosides and its preparation process
InactiveCN1602882AQuality improvementEasy to takeOrganic active ingredientsMetabolism disorderOrally disintegrating tabletPalpitations
The invention relates to a jiaogulan total saponin orally-disintegrating tablet, having the effects of reducing blood fat, improving myocardial anoxia and ischemia, etc, able to nourish heart and tonify spleen, benefit vital energy and promote blood circulation, etc, and applied to cure high lipemia and its preparing method, deficiency of vital energy such as palpitation and being short of breath, choking sensation in chest and limb anaesthesia, phlegm blocking and blood stagnation, and other symptoms, and its preparing technique. The invention provides a convenient-taken, quickly absorbed and taking-effect jiaogulan total saponin orally-disintegrating tablet and its preparing technique, using jiaogulan total saponin as raw material, using filling agent, disintegrant, flavor correcting agent, flowing aid, lubricant, etc as auxiliaries, able to use adhesive or coating according to different conditions and also able to add in a proper amount of effervescing agent according to the conditions, preparing by specific preparing method, and pressing into tablets by tablet presser and making it. It has the characters of good brittleness, quick disintegration, good taste, low production cost, convenience for carrying, storing, transporting, taking, etc; especially, it can be taken without water and quickly take effect, thus improving the compliance of patients and strengthening curative effect of drug.
Owner:COSCI MED TECH CO LTD
Palonosetron hydrochloride orally disintegrating tablet and preparation method thereof
ActiveCN102048705ALow friabilitySimple preparation processOrganic active ingredientsDigestive systemManufacturing technologyOrally disintegrating tablet
The invention belongs to the field of pharmacy and medical technology, and relates to a palonosetron hydrochloride orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet comprises the following components in percentage by mass: palonosetron hydrochloride 0.16 to 1.5, mannite 70 to 95, disintegrating agent 1.5 to 15, taste correcting agent 1 to 3, lubricating agent 0.5 to 2, and other auxiliary materials 0 to 15. The manufacturing technology can adopt dry direct tablet compressing. The palonosetron hydrochloride orally disintegrating tablet has the advantages of simple manufacturing technology, low cost and quick effect on indications, is convenient to take, can be disintegrated into fine grains or powder in oral cavity quickly after oral administration, and is particularly suitable for patients who swallow hard and tumor patients with weak physical fitness. The preparation exists in a form of fine grain or powder before reaching the gastrointestinal tract, and the medicine dissolves out in an accelerating manner and is distributed in the gastrointestinal tract in a large area for more absorption points, so that the bioavailability can be improved.
Owner:QILU PHARMA HAINAN
Precription of traditional Chinese medicine for treating gynaecological disease, its use and preparation method
The invention discloses a Chinese traditional medicine for curing women diseases and its prescription, application and the manufacturing method for the medicine. It is made up of semen coicis, sargentg loryvine, herba patriniae, bark of Chinese corktree, atractylodes rhizome, bamboo brier. The medicines is matched with accessories and flavor correcting agent and processed into particle; the medicine can be taken orally for curing chronic pelvic inflammatory disease, annexitis, endometritis, and it has functions of heat relieving and blood activating.
Owner:DUODUO PHARMA
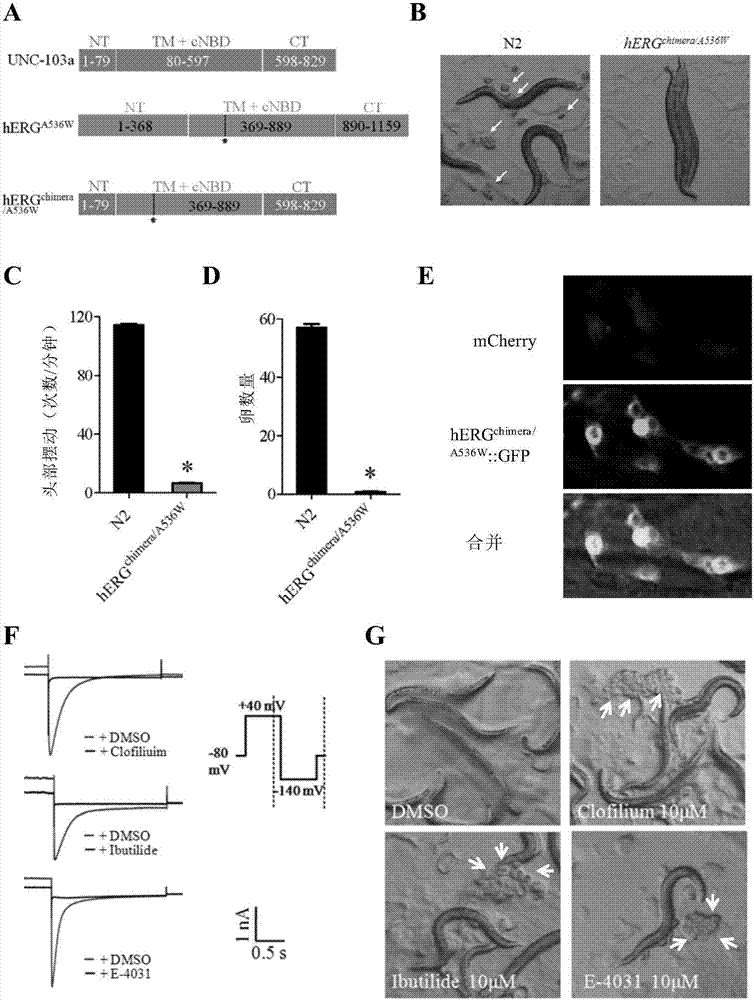
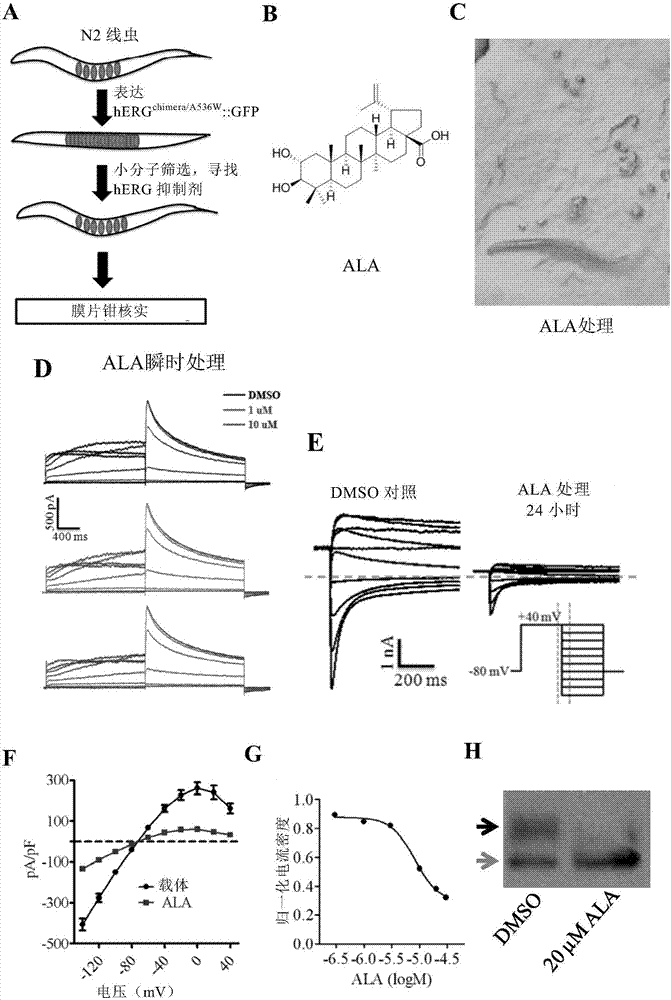
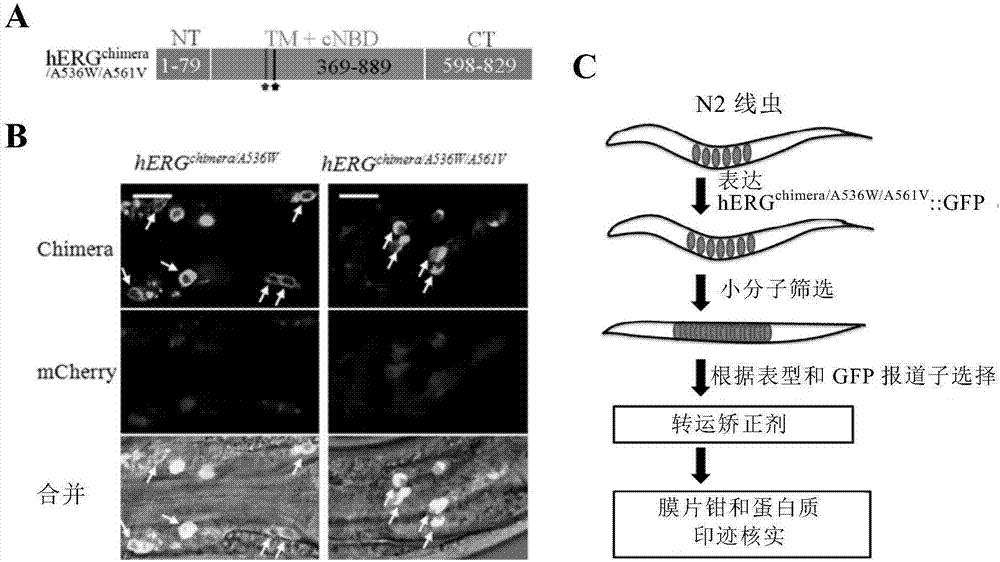
Method for screening hERG potassium ion channel agonist and detecting toxicity
ActiveCN107011444APolypeptide with localisation/targeting motifBiological testingCyclic nucleotide bindingScreening method
The invention relates to a method for screening hERG potassium ion channel agonist and detecting toxicity. Particularly, the invention firstly relates to a fusion protein, which contains a fragment (which is used as the N end of the fusion protein, is from a position between 1st to 85th amino acid residues of the N end of the nemathelminth ERG family potassium ion channels UNC-103 protein and has the length of 75 to 85 amino acid residues), hERG or a fragment at least containing S1-S6 transmembrane domains and cyclic nucleotide binding domains, and a fragment (which is used as the C end of the fusion protein, is from a position between 590th to 829th of amino acid residues of the C end of the UNC-103 protein and has the length of 220 to 240 amino acid residues). The invention also relates to a polynucleotide sequence coding the fusion protein, a relevant transgenic nemathelminth, a relevant screening method and application. The inventor builds an in-vivo hERG-intracellular-transport-influence-recognizable compound molecule screening method for screening hERG inhibitors or LQTS (long QT syndrome) relevant channel mutant functional correcting agents for the first time; the novel path and method are provided for hERG toxicity detection and LQTS treatment medicine screening.
Owner:CENT FOR EXCELLENCE IN BRAIN SCI & INTELLIGENCE TECH CHINESE ACAD OF SCI
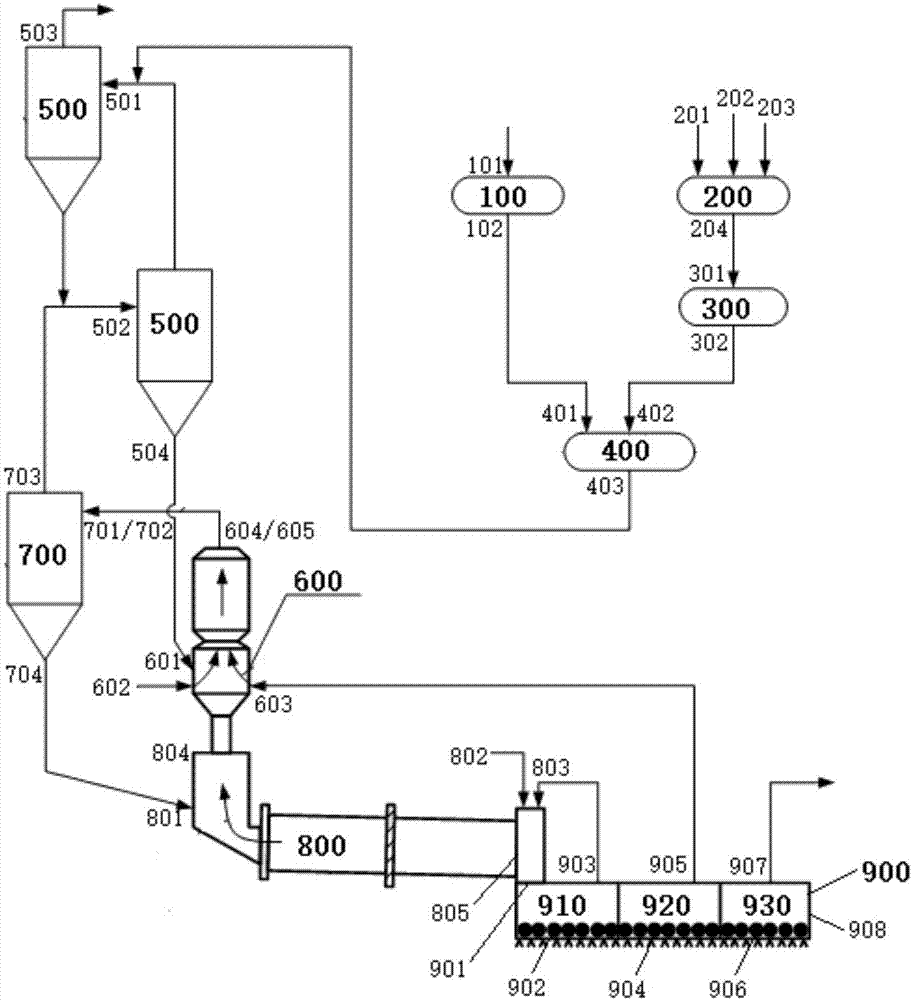
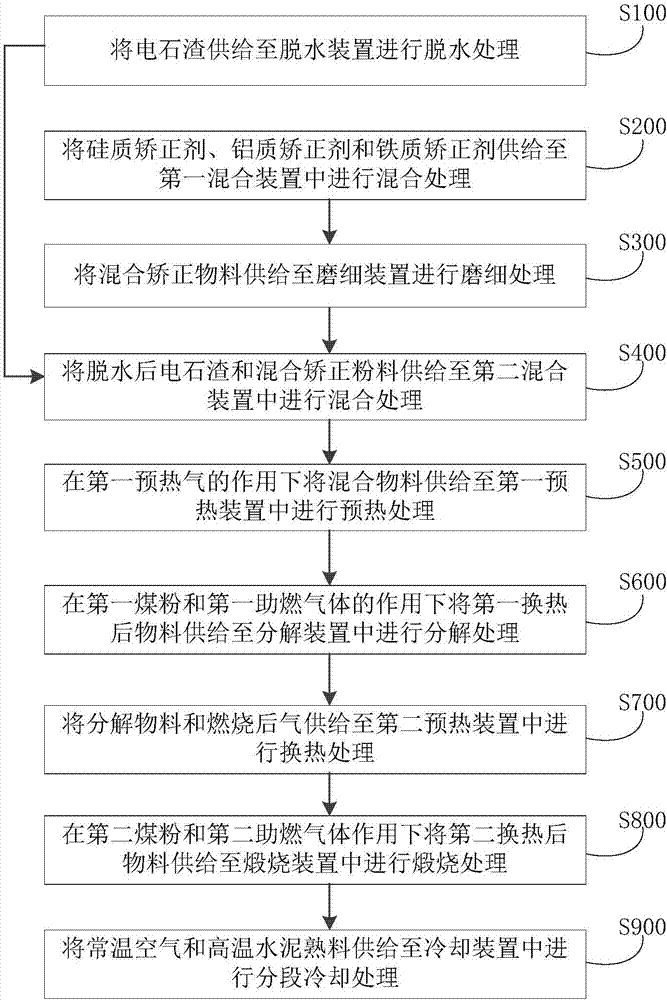
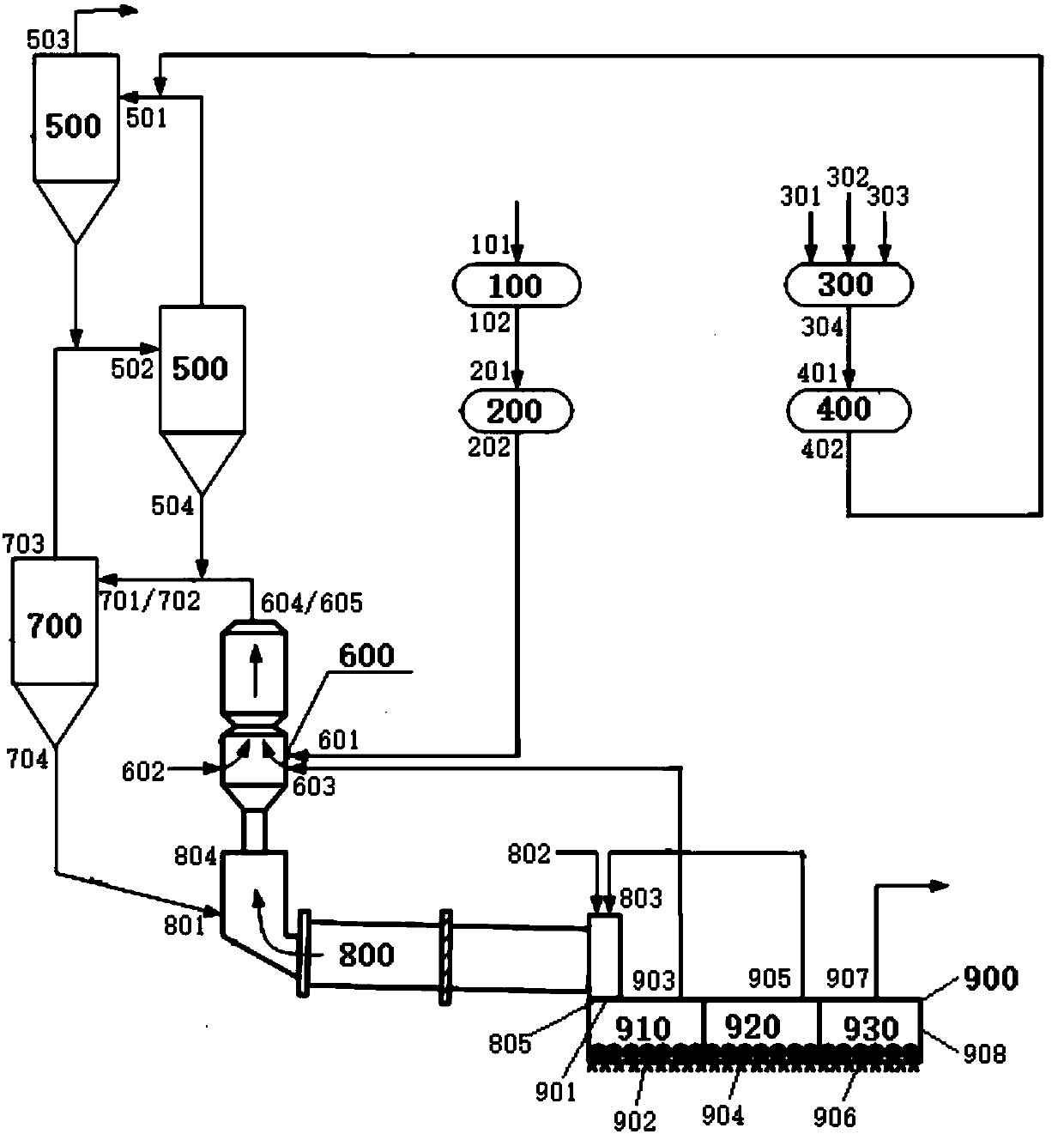
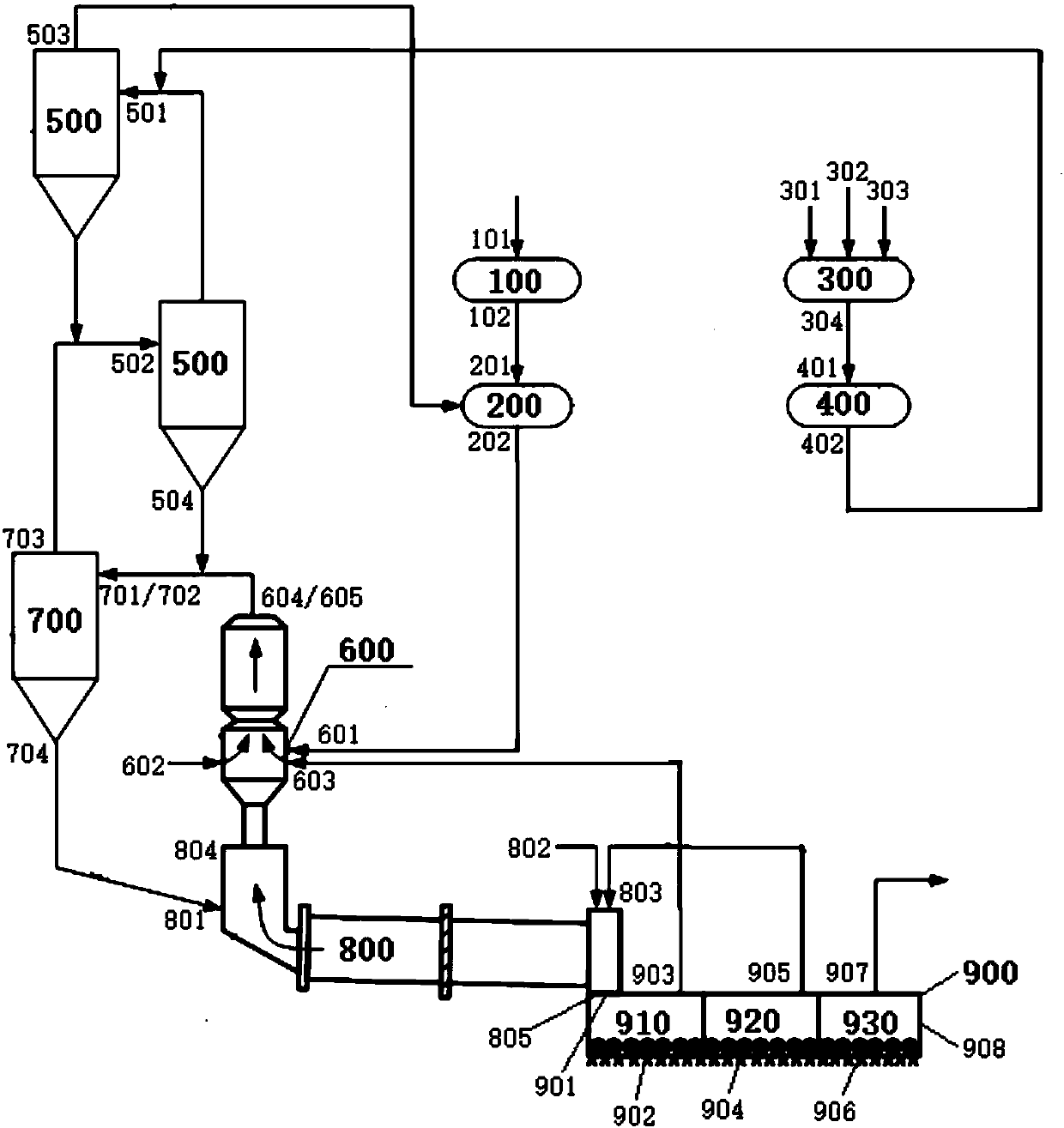
Cement clinker preparation system and method
The invention discloses a cement clinker preparation system and method. The system comprises a dewatering device, a first mixing device, a fine grinding device, a second mixing device, a first preheating device, a decomposition device, a second preheating device, a calcination device and a cooling device, wherein the dewatering device is provided with a carbide slag inlet and a dewatered carbide slag outlet; the first mixing device is provided with a silicon correcting agent inlet, an aluminum correcting agent inlet, an iron correcting agent inlet and a mixed correcting material outlet; the fine grinding device is provided with a fixed correcting material inlet and a mixed correcting powder outlet; the second mixing device is provided with a dewatered carbide slag inlet, a mixed correcting power inlet and a mixed material outlet; the first preheating device is provided with a mixed material inlet and a first heat exchange material outlet; the decomposition device is provided with a first heat exchange material inlet and a decomposition material outlet; the second preheating device is provided with a material inlet and a second heat exchange material outlet; the roasting device is provided with a second heat exchange material inlet and a high-temperature cement clinker outlet; the cooling device is provided with a high-temperature cement clinker inlet, a first gas outlet, a second gas outlet, a third gas outlet and a cement clinker outlet.
Owner:JIANGSU PROVINCE METALLURGICAL DESIGN INST
Binder modified with glycerol for making asphalt mixes with a hicontent of recycled bituminous materials
InactiveUS9115284B2Improve compactabilityImprove performanceSolid waste managementBituminous material adhesivesGlycerolBituminous materials
Owner:COLAS LTD
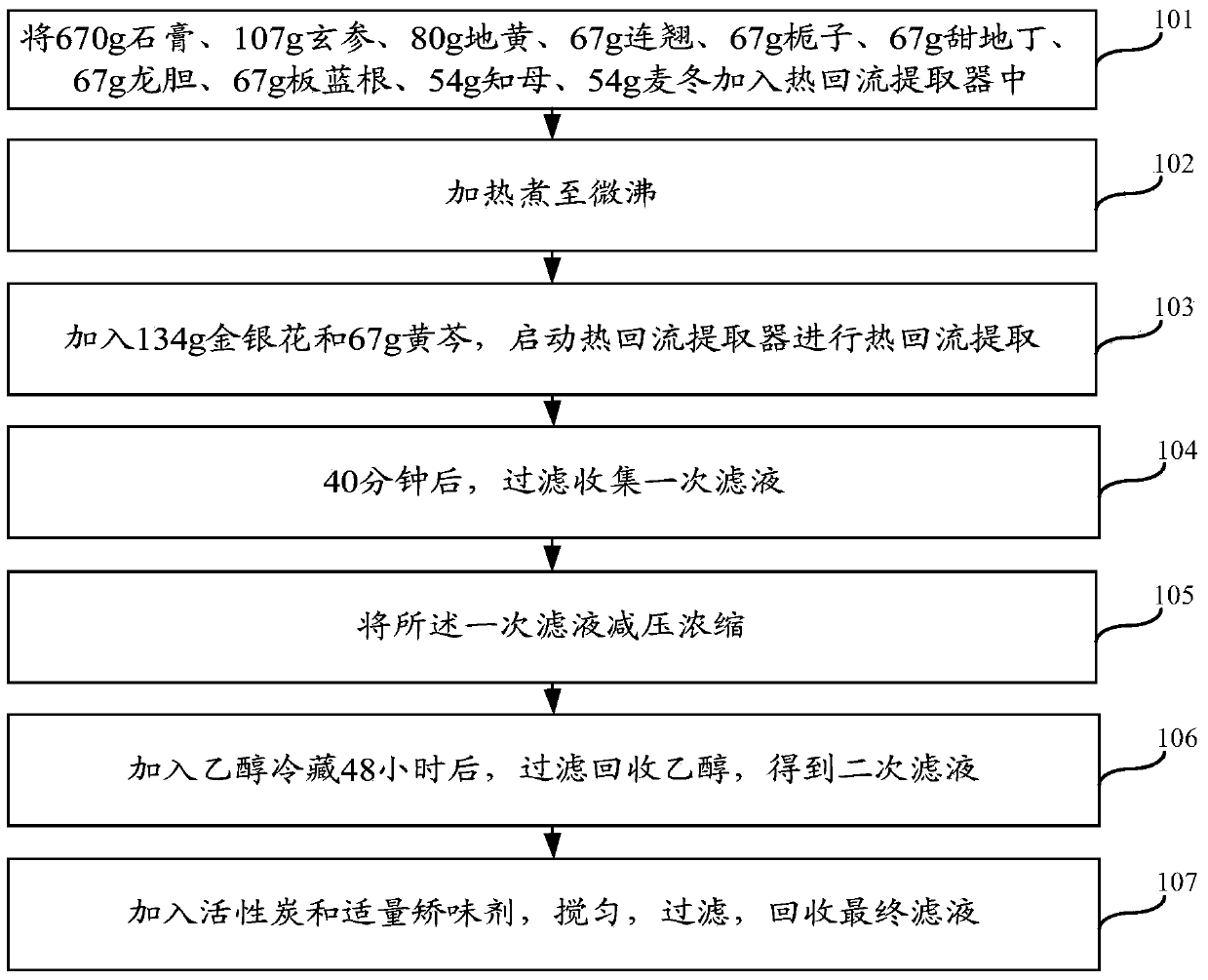
Preparation method of heat-clearing and detoxifying oral liquid
ActiveCN103735831AReduce precipitationSolve the process problem of difficult filtrationAluminium/calcium/magnesium active ingredientsPlant ingredientsBULK ACTIVE INGREDIENTRadix Ophiopogonis
The invention relates to the field of pharmacy, in particular to a preparation method of a heat-clearing and detoxifying oral liquid. The oral liquid comprises the following steps of adding 670g of gypsum, 107g of radix scrophulariae, 80g of rehmannia, 67g of fructus forsythiae, 67g of jasmine, 67g of gueldenstaedtia multiflora bunge, 67g of radix gentianae, 67g of radix isatidis, 54g of rhizoma anemarrhenae, and 54g of radix ophiopogonis into a heat reflux extractor; heating to slightly boil; adding 134g of honeysuckle and 67g of radix scutellariae, and starting the heat reflux extractor to carry out heat reflux extraction; after 40 minutes, filtering and collecting primary filtrate; depressurizing and concentrating the primary filtrate; adding ethanol to refrigerate for 48 hours, and filtering and recovering the ethanol, so as to obtain secondary filtrate; adding active carbon and a proper amount of flavor correcting agent, uniformly mixing, filtering, and recovering the final filtrate. The preparation method has the advantages that as the extraction time is shortened, the segregation of inactive ingredients, such as tannin and pigments, in medicine liquor is reduced, and the process problem of difficult medicine liquor filtering is solved; by increasing the content of active ingredient of baicalin, the medicine effect is greatly improved under the condition of same dosage amount.
Owner:JILIN JINFUKANG PHARMA
Chrysanthemum planting method
InactiveCN106688529APromote growthInhibition of germinationExcrement fertilisersPlant cultivationDiseaseEconomic benefits
The invention discloses a chrysanthemum planting method. According to the technical scheme, the method sequentially includes the steps of firstly, selecting parent body, and sterilizing and rinsing the parent body; secondly, cultivating at an appropriate position; thirdly, pruning, and performing cuttage; fourthly, performing correction with a correcting agent after seedling recovering of chrysanthemum; fifthly, controlling diseases and pests; sixthly, transplanting. The method has the advantages that invasion of bacteria and viruses into plants can be inhibited, the diseases and pests can be resisted, the chrysanthemum can grow fast, high survival rate is achieved, and economic benefits are increased.
Owner:曹才基
Boletic acid quetiapine oral preparation and preparation method thereof
InactiveCN101375852ADisintegrates quicklyFast absorptionOrganic active ingredientsNervous disorderQuetiapineDrug administration
The invention discloses a quetiapine fumarate oral preparation and a preparation method thereof. The quetiapine fumarate oral preparation is mainly prepared by raw materials with the following proportion by weight: 25-300 parts of quetiapine fumarate counted by quetiapine, 2-100 parts of disintegrating agent and 5-400 parts of filling agent. The preparation method of the oral preparation comprises the following process steps: (1) the quetiapine fumarate is taken, smashed, screened and evenly mixed with flavoring agent and the disintegrating agent, and wetting agent is added for preparing soft materials; (2) the soft materials are screen through a 20-mesh sieve for carrying out the granulation and the drying, the 20-mesh sieve is used for carrying out the size stabilization, the filling agent, an odor correcting agent, glidant and lubricant are added for even mixing; (3) the content of the drug which is evenly mixed is measured, the tablet weight is calculated, and the tablet pressing is carried out for the preparation. The oral preparation can be rapidly disintegrated in oral cavity without the use of water for the drug administration; the absorption is rapid and the bioavailability is high; and the taste is good.
Owner:张宏宇
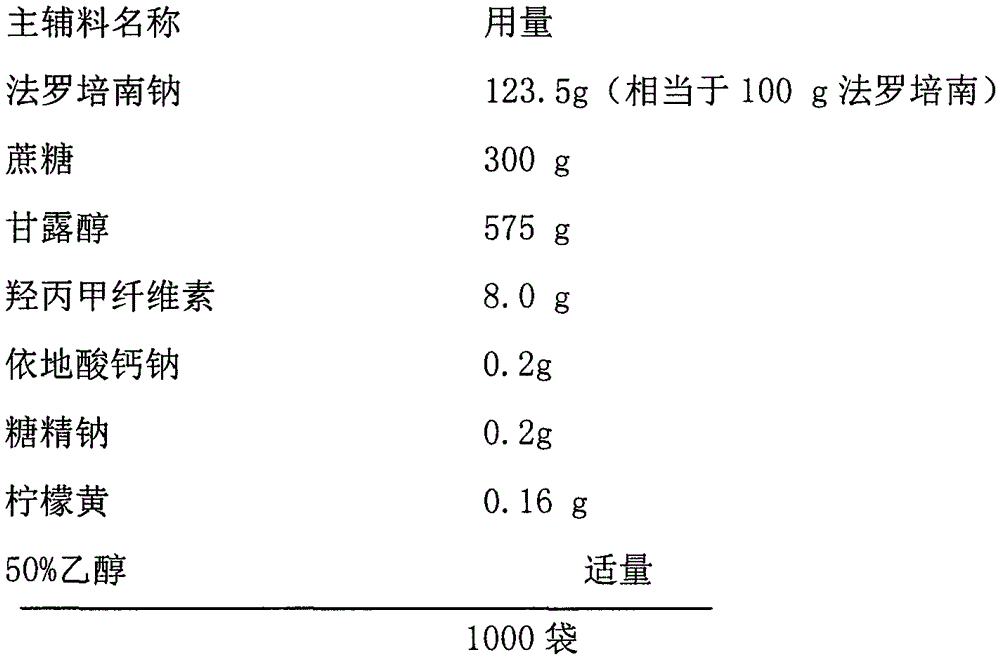
Faropenem sodium granules
InactiveCN106667917AHigh hardnessGood water solubilityAntibacterial agentsPharmaceutical non-active ingredientsSolubilitySide effect
The invention provides faropenem sodium granules. The faropenem sodium granules are characterized by being prepared from the following components in parts by weight: 100 parts of faropenem sodium calculated on the basis of faropenem, 300 to 400 parts of saccharose, 400 to 575 parts of mannitol, 8.0 to 80 parts of hydroxypropyl methylcellulose, 0.1 to 0.2 part of sodium calcium edentate, 0 to 50 parts of a taste correcting agent and 0 to 0.2 part of a color correcting agent. The prepared faropenem sodium granules have good granule hardness, high water solubility and moderate taste and color, solve the defect of low stability of the conventional granules, and are stable in release, durable in action, stable in curative effect, small in toxic and side effect and particularly favorable for children and people suffering from dysphagia to take.
Owner:HAINAN HONZ PHARMA
Preparation method of tobacco sheet shreds with banana taste
InactiveCN103637394AMild and Comfortable TasteModerate sensory stimulationTobacco preparationTobacco treatmentAlcoholAdhesive
The invention discloses a preparation method of tobacco sheet shreds with banana taste. Tobacco powder, adhesives, water and adsorbents containing banana alcohol extract are mixed to form tobacco sheet slurry, the mass ratio of the adsorbents containing the banana alcohol extract in the tobacco sheet slurry is 23%-30%, the mass ratio of the tobacco powder in the tobacco sheet slurry is 15%-25%, the mass ratio of the adhesives in the tobacco sheet slurry is 1%-5%, the mass ratio of sweetening agents in the tobacco sheet slurry is 3%-5%, and the mass ratio of correcting agents in the tobacco sheet slurry is 0.1%-0.35%. The tobacco sheet shreds prepared by the method have intense banana taste, are gentle and comfortable in taste, moderate in sensory stimulation and suitable for consumption psychology and taste characteristics of domestic consumers, and can provide satisfaction similar to that of traditional cigarettes and foreign existing mouth insertion cigarettes for tobacco consumers.
Owner:TOBACCO SCI RES INST CHONGQING CITY COMPANY OF CHINA NAT TOBACCO
Dracocephalum moldavica general flavone orally disintegrating tablet and preparation method thereof
ActiveCN102048810AGreat tasteDisintegrates quicklyPill deliveryCardiovascular disorderDiseaseDracocephalum moldavica
The invention discloses a dracocephalum moldavica general flavone orally disintegrating tablet and a preparation method thereof. The dracocephalum moldavica general flavone orally disintegrating tablet is prepared by dosing, pelletizing and tabletting 25 to 30 portions of dracocephalum moldavica general flavone, 9 to18 portions of disintegrating agents, 33 to 85 portions of filling agents, 1 to 5 portions of binding agents, 0.5 to 1 portions of lubricant, and 1 to 3 portions of taste correcting agents. Compared with the prior art, the dracocephalum moldavica general flavone orally disintegrating tablet is a novel preparation with favorable taste and quick disintegration, has the characteristics of convenience in taking and quickness in taking effect, can solve the problem of the medicine taking of patients who swallow difficultly, can meet the requirement for the patients who take medicine without water, overcomes the problem of insufferable bitter taste of other tablets without taste correction, improves the conformance of patients, has the characteristic of quickness in disintegration, and can disintegrate within 15 to 60 seconds in the oral cavity once being contacted with saliva, thereby being an ideal pharmaceutical preparation for preventing arteriosclerosis, coronary heart disease and angina pectoris disease in a clinic.
Owner:XINJIANG BIOCHEM PHARMA CO LTD
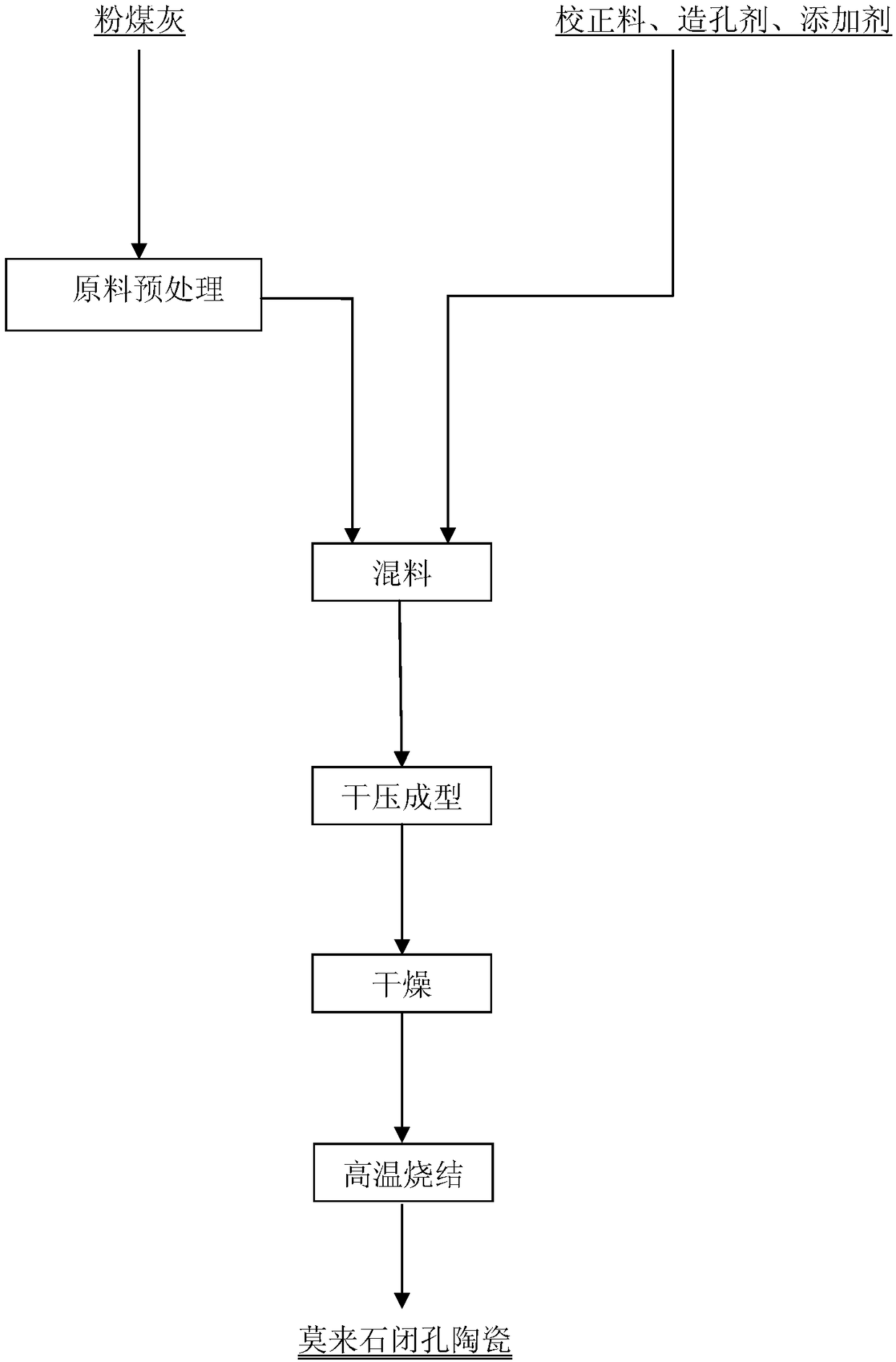
Fly ash-based mullite closed-cell ceramic and preparation method thereof
The invention provides fly ash-based mullite closed-cell ceramic and a preparation method thereof. The closed-cell ceramic is prepared from the following components: fly ash clinker, a correcting agent, a pore forming material and a fluxing agent in a mass ratio of 100:(100-200):(0-15):(10-20). The preparation method comprises the following steps: 1, calcining fly ash to obtain the fly ash clinker; 2, uniformly mixing the raw materials in the mass ratio to prepare a uniform mixture; 3, performing dry-pressing molding on the uniform mixture, sufficiently drying the molded material, sintering ina high-temperature furnace, and insulating for a period to prepare the fly ash-based mullite closed-cell ceramic. The fly ash-based mullite closed-cell ceramic is economical and environment-friendlyin raw material application, has a simple and feasible method operation and is convenient for industrial production, the closed porosity of the mullite closed-cell ceramic can be improved, the thermalconductivity of the ceramic can be reduced, and the tensile strength of the mullite ceramic can be improved. The fly ash-based mullite closed-cell ceramic has excellent comprehensive performance andwide application prospect.
Owner:NORTHEASTERN UNIV
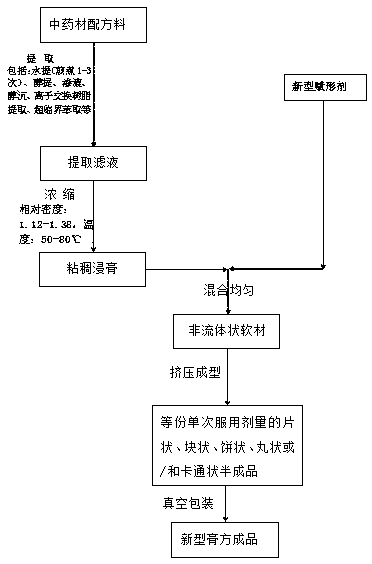
Novel cream formula production method
PendingCN109105734AMeet needsRealize no more sufferingPharmaceutical delivery mechanismPharmaceutical non-active ingredientsFlavorMedicine
The invention discloses a novel cream formula production method, which belongs to the field of traditional Chinese medicine treatment cream formula and sub-health regulation and healthcare cream formula as well as field of healthcare food and food. The novel cream formula production method comprises the following steps: S1, adding Chinese herb formula materials into an extraction device to performan appropriate extraction process, merging and filtering an obtained extraction solution, and obtaining filtrate for standby use; S2, concentrating the filtrate into viscous extract, wherein the relative density of the viscous extract is 1.12 to 1.38; S3, adding a novel shape forming agent into the viscous extract, and uniformly mixing to obtain a non-fluid soft material; and S4, extruding molding the non-fluid soft material into an equal amount of semi-finished product for a single administration dosage, and vacuum packaging, thus obtaining a novel cream formula finished product. By utilizing the novel shape forming agent, the taste of the traditional Chinese medicine cream formula can be thoroughly improved without adding any flavor correcting agent, and richer nutritional substances can be provided; by adding no preservative, the novel cream formula is healthier to the human body; and the novel cream formula is accurate in administration dose and easy to store and carry.
Owner:葛强
Donky-hide blood-supplementing tonic and its preparation method
InactiveCN1267124CDefinite curative effectEasy to useCarbohydrate active ingredientsUnknown materialsSide effectPreservative
The invention discloses an ass-hide glue blood enriching oral liquid and its manufacturing method which relates to a blood enriching medicine with Chinese traditional medicine as materials and its manufacturing method. The ass-hide blood enriching oral liquid uses ass-hide glue, prepared rehmannia root, co donopsis pilosula root, astragalus, medlar, lagehead atractylodes, taste correcting agent, aseptic as materials, and the oral liquid can be produced with current processing technology, the effect is accurate through several years of clinic experiences, the blood enriching effect is prominent, and it has no toxin and side effects.
Owner:FUJIAO GROUP CORP SHANDONG
Combination therapy for the treatment and improvement of scars
InactiveUS20070265346A1Good lookingSmall sizeBiocideCosmetic preparationsClose woundsTissue remodeling
The present invention is a composition, methods of using that composition and kits including that composition, useful for reducing the size and improving the appearance of a closed wound wherein the composition comprises a therapeutically effective amount of a hydrophilic or hydrophobic carrier (or a mixture thereof), at least one matrix metalloproteinase (MMP) modulator in combination with one or more of the following pharmaceutically active agents: (a) cell cycle modulators; (b) inflammatory event modulators; (c) angiogenesis event modulators; (d) fibroblast migration agents; (e) fibroblast proliferation agents; (f) tissue remodeling correcting agents; (g) antimicrobial agents; (h)modulators of deposition of extra cellular matrix; (i) penetration enhancers; (j) antioxidants; (k) antipuritic agents; (l) fibrinolytic agents; (j) immunomodulators; (m) transcription modulating agents; (n) surface modulating agents; (o) growth factor inhibitors; and (p) anti-proliferative agents.
Owner:AVOCET POLYMER TECH
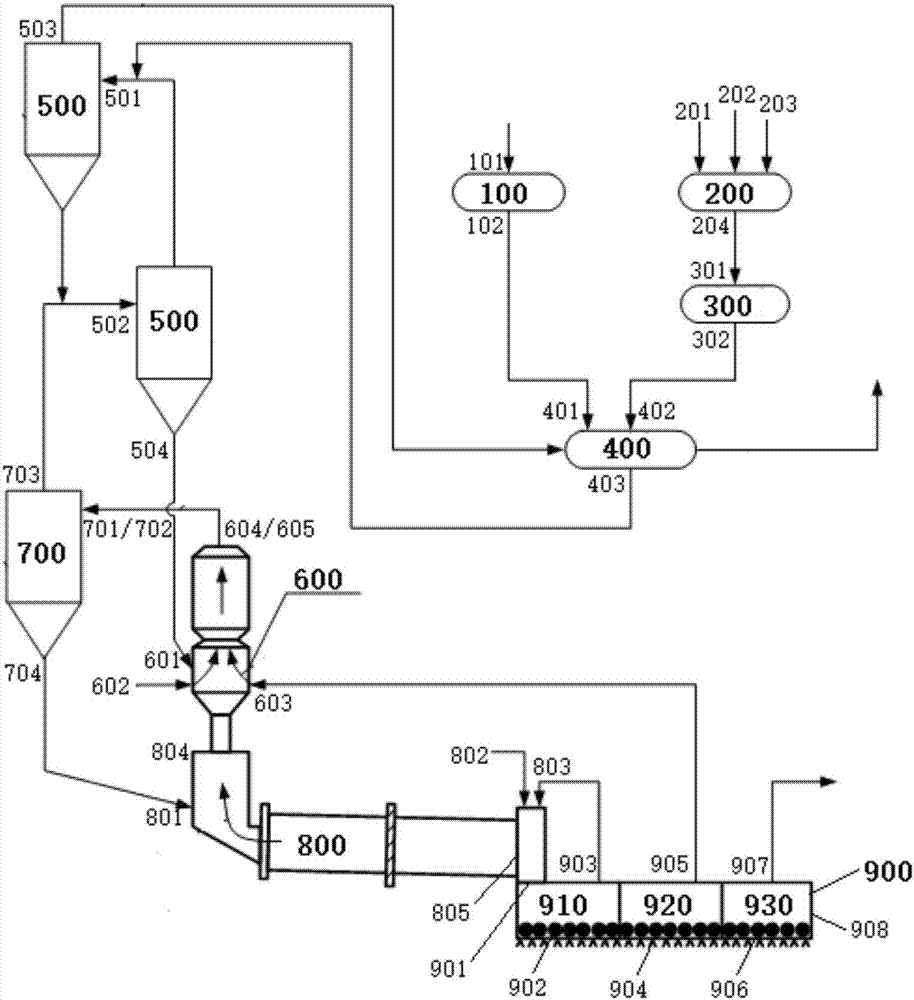
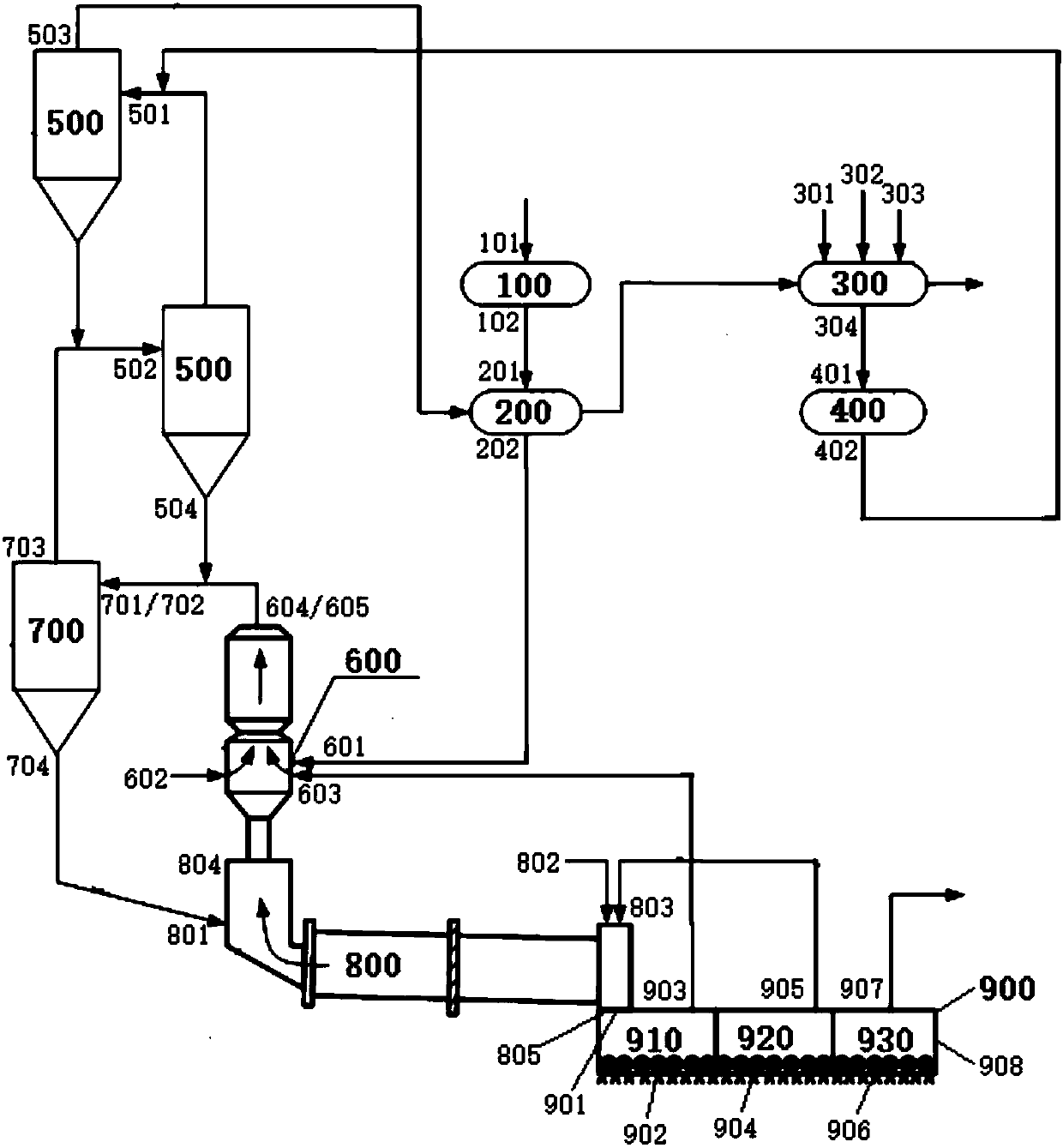
System and method for preparing cement clinker
The invention discloses a system and method for preparing cement clinker. The system comprises a dewatering device, a drying crusher, a mixing device, a grinding device, a first preheating device, a decomposing device, a second preheating device, a calcination device and a cooling device; and the dewatering device has a carbide slag inlet and a dehydrated carbide slag outlet, the drying crusher has a dehydrated carbide slag inlet and a dry carbide slag particle outlet, the mixing device has a silicon based correcting agent inlet, an aluminum based correcting agent inlet, an iron based correcting agent inlet and a mixed correction material outlet, the grinding device has a mixed correction material inlet and a mixed correction powder material outlet, the first preheating device has a mixedcorrection powder material inlet and a first-preheated material outlet, the decomposing device has a dry carbide slag particle inlet and a decomposed material outlet, the second preheating device hasa material inlet and a second-heat-exchanged material outlet, the calcination device has a second-heat-exchanged material inlet and a high-temperature cement clinker outlet, and the cooling device hasa high-temperature cement clinker inlet and a cement clinker outlet.
Owner:JIANGSU PROVINCE METALLURGICAL DESIGN INST
Medicine for animals for treating and preventing avian influenza
InactiveCN101062405AActivation of responsivenessRaise antibody levelsPeptide/protein ingredientsAntiviralsFowlCurative effect
The invention discloses an animal medicine to prevent and treat fowl flu, which is characterized by the following: incorporating 500-5000mg alpha-mannan peptide solution per 1000ml; proceeding space embarkation for optimization alpha-hemoclastic streptococcus 33# bacterial; proceeding space mutagenesis; getting strain; using as production bacterial; proceeding one grade ferment and two grade ferment; producing mannan peptide zymotic fluid; purifying the zymotic fluid; filtering; adding proper essence and correcting agent; dissolving; formulating; stirring evenly; filtering; loading; sealing; sterilizing at hot pressure; getting ferment stock solution. This invention possesses high curative effect without drug tolerance.
Owner:西安亨通光华制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com