O-aminobiphenyl compound synthesis method
A technology for o-aminobiphenyl and compounds, which is applied in the field of synthesizing o-aminobiphenyl compounds, can solve the problems of difficult industrial acquisition of raw materials, high preparation or high price, harsh reaction conditions, etc., and achieves reduced production costs, simple operation, and comprehensive synthetic routes short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
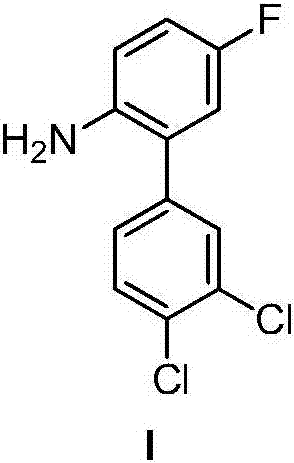
[0063] Example 1 3',4'-Dichloro-2-amino-5-fluorobiphenyl
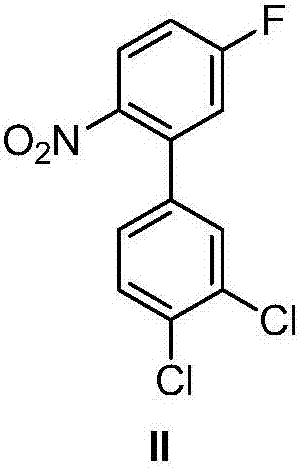
[0064] (1) Preparation of 2-nitro-5-fluorobenzoate
[0065] Add 111.0g of 2-nitro-5-fluorobenzoic acid (with a mass percentage of 99%, within 100g directly from Huaian Ping An Chemical Co., Ltd., and prepare a large amount according to the method described in US 5591890) in a 2L three-necked bottle, 600mL without Water ethanol, add potassium hydroxide ethanol solution dropwise at room temperature (38.9g of 85% by mass potassium hydroxide dissolved in 600mL absolute ethanol), stir at room temperature for 2 hours, during this process a large amount of light yellow solid precipitates , Filtered and dried to obtain 123.6 g of light yellow powdery solid potassium salt of 2-nitro-5-fluorobenzoic acid with a mass percentage of 99%.
[0066] (2) Preparation of 3',4'-dichloro-2-amino-5-fluorobiphenyl
[0067] Add 13.38 g (0.06 mol) 2-nitro-5-fluorobenzoic acid potassium salt, 0.22 g (0.0012 mol) cuprous iodide, 0.12 g (0.0004 mol) to ...
Embodiment 36
[0079] Into a dry 250 ml three-necked flask equipped with a magnetic stirrer were added 13.38 g of 2-nitro-5-fluorobenzoic acid potassium salt, 0.22 g of cuprous iodide, 0.12 g of palladium acetylacetonate, 0.36 g of 1,10 -Phenanthroline, 8.44 grams of 3,4-dichlorobromobenzene, 3 grams of 3A molecular sieve, and 80 grams of anhydrous polyethylene glycol PEG-400, and then nitrogen vacuum replacement three times, under the protection of nitrogen, stirring and heating to 190 ℃, react for 23 hours. Use gas chromatograph or high pressure liquid chromatograph to detect the end of the reaction, cool the reaction solution to room temperature, filter, add 120 ml of water to the filtrate to evenly dilute, extract 3 times with toluene (60 ml / time), combine the extracts and wash them with water (100 ml / time), the organic phase was concentrated with a rotary evaporator, the oily mixture obtained was recrystallized with petroleum ether, and after filtration, a yellow 3',4'-dichloro-2-amino-5...
Embodiment 37
[0081] Into a dry 250 ml three-necked flask equipped with a magnetic stirrer were added 13.38 g of 2-nitro-5-fluorobenzoic acid potassium salt, 0.22 g of cuprous iodide, 0.12 g of palladium acetylacetonate, 0.36 g of 1,10 -Phenanthroline, 8.44 grams of 3,4-dichlorobromobenzene, 3 grams of 3A molecular sieve, and 80 grams of anhydrous trimethylbenzene, and then nitrogen vacuum replacement three times, under the protection of nitrogen, stirring and heating to 190 ℃, reaction for 23 hours . After the reaction is detected by gas chromatograph or liquid chromatograph, the reaction liquid is cooled to room temperature, filtered, 120 ml of water and 150 ml of trimethylbenzene are added to the filtrate, and the mixture is stirred evenly. The organic phase is separated and washed with water (100 ml / time) The organic phase was concentrated twice with a rotary evaporator. The oily mixture obtained was recrystallized with petroleum ether. After filtration, 10.40 g of yellow 3',4'-dichloro-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com