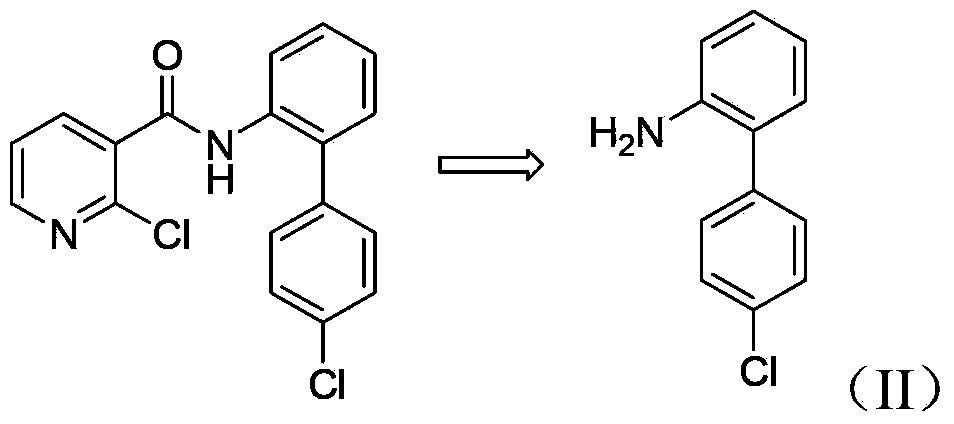
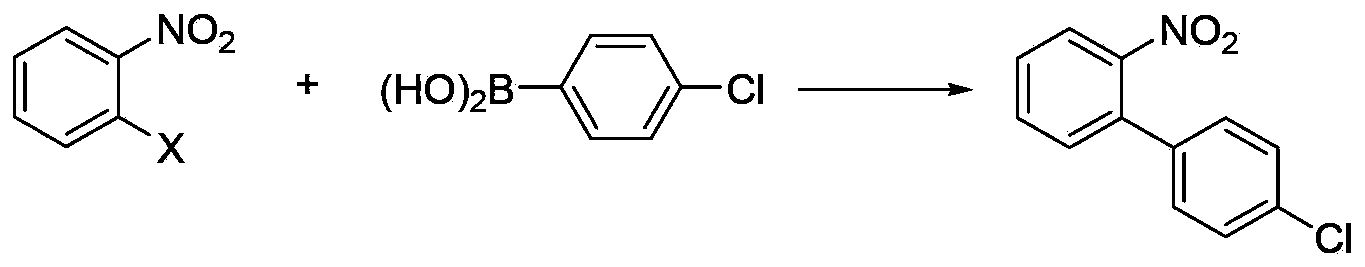
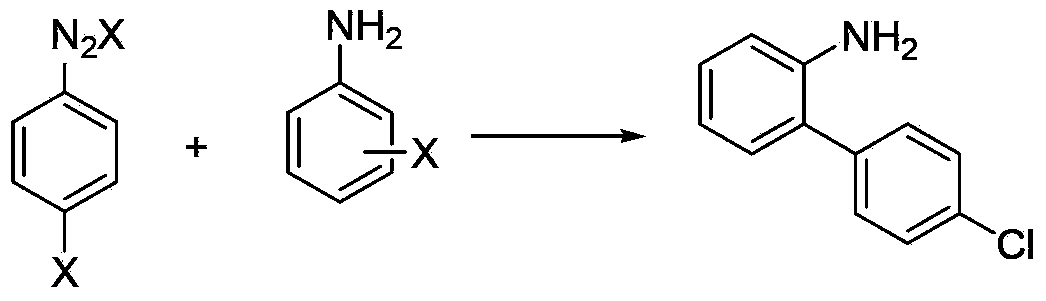Method for synthesizing nitrobiphenyl compound
A synthesis method and technology of nitrobiphenyl, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult industrialization, difficult acquisition of raw materials, low yield of products, etc., and achieve easy access, Avoidance of low temperature reactions, low technical and equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 10mL of nitrobenzene and 1.92g of p-chlorobromobenzene, 0.11g of palladium acetate, 0.262g of triphenylphosphine and 4.88g of cesium carbonate into a 50mL three-necked flask equipped with a reflux condenser and a stirring device. Heated to 140°C for 24 hours. After the reaction, insoluble matter was filtered off, nitrobenzene was distilled off under reduced pressure, 5 mL of ethanol was added to the crude product, and refluxed for 0.5 hours. Then the insolubles were filtered off, and the filtrate was cooled and crystallized to obtain 1.65 grams of yellow crystal 4-chloro-2'-nitrobiphenyl, with a yield of 67% and a purity of 95%. Characterized by NMR as the target product: 1 H NMR (300MHz, CDCl 3 ): δ=7.24(d,J=6.0Hz,2H),7.38-7.42(m,3H),7.50-7.51(m,1H),7.62-7.63(m,1H),7.86-7.89(m,1H ) ppm.
Embodiment 2
[0041] Add 10 mL of nitrobenzene, 2.36 g of 1,4-dibromobenzene, 0.11 g of palladium acetate, 0.262 g of triphenylphosphine and 4.88 g of cesium carbonate into a 50 mL three-necked flask equipped with a reflux condenser and a stirring device, Under atmospheric conditions, it was heated to 140° C. to react for 24 hours. After the reaction, insoluble matter was filtered off, nitrobenzene was distilled off under reduced pressure, 5 mL of ethanol was added to the crude product, and refluxed for 0.5 hours. Then the insolubles were filtered off, and the filtrate was cooled and crystallized to obtain 1.93 grams of yellow crystal 4-bromo-2'-nitrobiphenyl, with a yield of 66% and a purity of 95%. Characterized by NMR as the target product: 1 H NMR (300MHz, CDCl 3 ): δ=7.23(d,J=6.0Hz,2H),7.37-7.40(m,3H),7.48-7.50(m,1H),7.61-7.63(m,1H),7.85-7.90(m,1H ) ppm.
Embodiment 3
[0043] Add 10 mL of nitrobenzene and 2.61 g of (4-bromophenyl) phenyl ketone, 0.11 g of palladium acetate, 0.262 g of triphenylphosphine and 4.88 g of carbonic acid in a 50 mL three-necked flask equipped with a reflux condenser and a stirring device cesium, heated to 140°C for 24 hours under nitrogen atmosphere. After the reaction, insoluble matter was filtered off, nitrobenzene was distilled off under reduced pressure, 5 mL of ethanol was added to the crude product, and refluxed for 0.5 hours. Then the insolubles were filtered off, and the filtrate was cooled and crystallized to obtain 2.25 grams of yellow crystal 4-benzoyl-2'-nitrobiphenyl, with a yield of 70% and a purity of 94%. Characterized by NMR as the target product:1 H NMR (300MHz, CDCl 3 ): δ=7.40-7.53(m,6H),7.55-7.59(m,1H),7.62-7.66(m,1H),7.84(t,J=8.4Hz,4H),7.89-7.95(m,1H ) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com