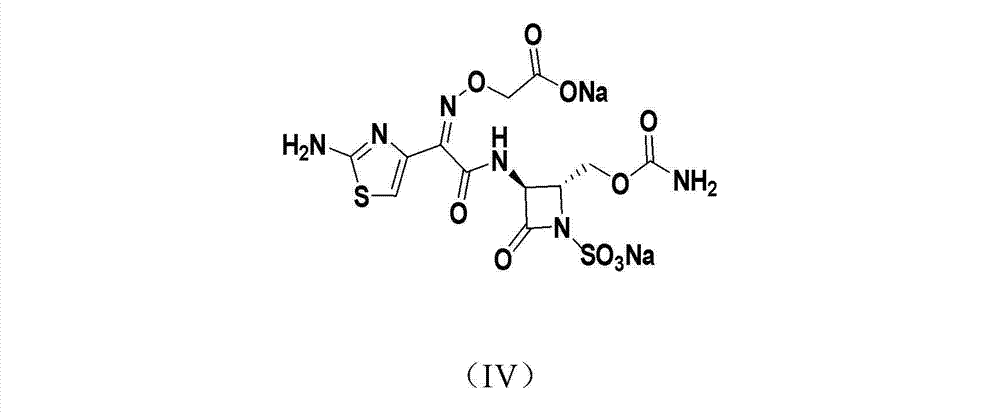
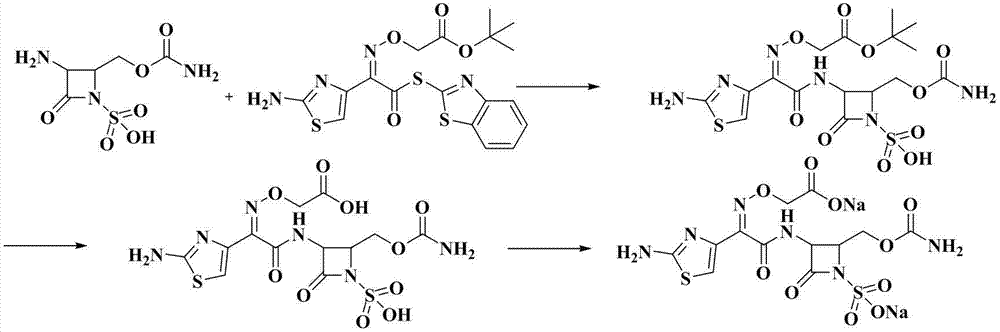
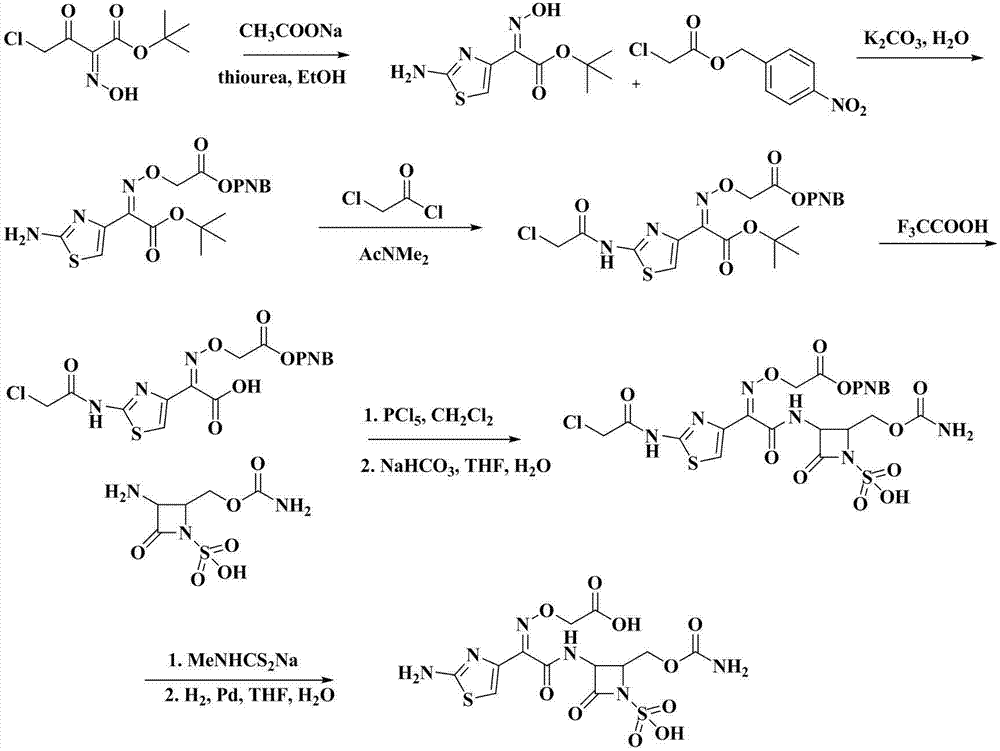
Synthetic method of Carumonam sodium
A synthesis method and organic solvent technology are applied in the synthesis field of calumonant sodium, can solve the problems of complicated steps, low yield and the like, and achieve the effects of simple operation, lower production cost, and avoidance of protection and deprotection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In a 500mL three-neck flask, add (3S-trans)-3-amino-2-(carbamoyloxymethyl)-4-oxoazetidinesulfonic acid (23.9g, 0.1mol), dichloro 180mL of methane was stirred and dissolved, the temperature was lowered to 0-5°C, and triethylamine (40g, 0.4mol) was started to be added dropwise, while (Z)-2-(1-(2-aminothiazol-4-yl)- 2-Chloro-2-oxoethyleneaminooxy) acetic acid hydrochloride (30.0g, 0.1mol), control the reaction temperature at 0-5°C, pH to 7-8, after adding, keep warm at 0-5°C React for 3 hours, add 300g of purified water, stir for 15 minutes, statically separate into layers to obtain a water layer, add 2g of activated carbon to the water layer, stir for 20 minutes to decolorize, filter with suction, and adjust the pH value of the filtrate with 37.5wt% concentrated hydrochloric acid at 0-5°C 1.5 to 2.0, keep warm and stir for crystallization for 2 hours, filter with suction to get the wet product of Kalumonan.
[0046] In a 1000mL three-necked flask, add sodium acetate (18....
Embodiment 2
[0048] In a 500mL three-necked flask, add (3S-trans)-3-amino-2-(carbamoyloxymethyl)-4-oxoazetidinesulfonic acid (23.9g, 0.1mol), ethyl acetate 180ml of the ester was stirred and dissolved, the temperature was lowered to -5~0°C, and pyridine (23.7g, 0.3mol) was added dropwise, while (Z)-2-(1-(2-aminothiazol-4-yl)- 2-Chloro-2-oxoethyleneaminooxy) acetic acid hydrochloride (45.0g, 0.15mol), control the reaction temperature -5~0℃, pH to about 5~6, after adding, -5~ The reaction was carried out at 0°C for 3 hours, and the post-treatment was the same as in Example 1 to obtain the wet product of Kalumonan.
[0049] In a 1000mL three-neck flask, add sodium hydroxide (11.2g, 0.28mol) and 100ml of purified water, stir to dissolve, add the wet product of calumonan obtained above, stir and react at 0-10°C for 3 hours, add absolute ethanol dropwise 500ml, cooled to -20~-15°C, stirred and crystallized for 2 hours, suction filtered, and the filter cake was vacuum-dried at 40°C to obtain 36....
Embodiment 3
[0051] In a 500mL three-neck flask, add (3S-trans)-3-amino-2-(carbamoyloxymethyl)-4-oxoazetidinesulfonic acid (23.9g, 0.1mol), dichloro 180ml of methane was stirred and dissolved, the temperature was lowered to -20~-15°C, piperidine (31.6g, 0.4mol) was added dropwise, and (Z)-2-(1-(2-aminothiazol-4-yl )-2-Chloro-2-oxoethyleneaminooxy)acetic acid hydrochloride (36.0g, 0.12mol), control the reaction temperature -20~-15℃, pH to about 8~9, after adding, -20~-15°C heat preservation reaction for 3 hours, post-treatment as in Example 1, to obtain Kalumonan wet product.
[0052] In a 1000mL three-neck flask, add sodium carbonate (53g, 0.5mol) and 130ml of purified water, stir to dissolve, add the wet product of carumonan obtained above, stir and react at 40-50°C for 1 hour, add dropwise 300ml of tetrahydrofuran, and cool down to Stir and crystallize at -5 to 0°C for 2 hours, filter with suction, and vacuum-dry the filter cake at 60°C to obtain 37.3 g of calumonan sodium, with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com