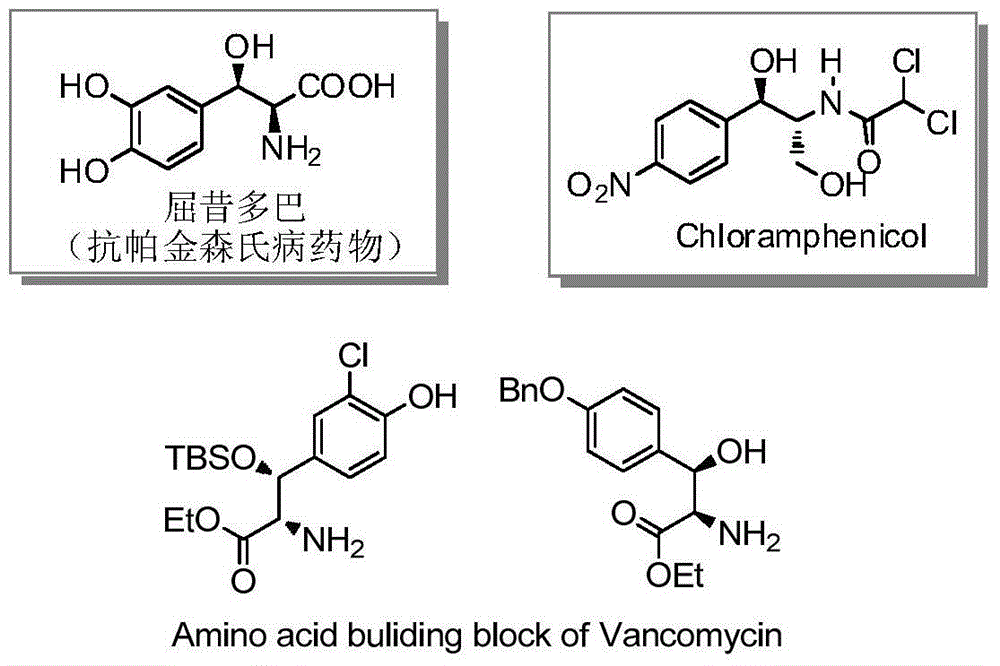
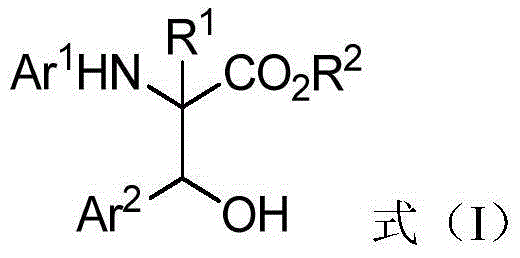
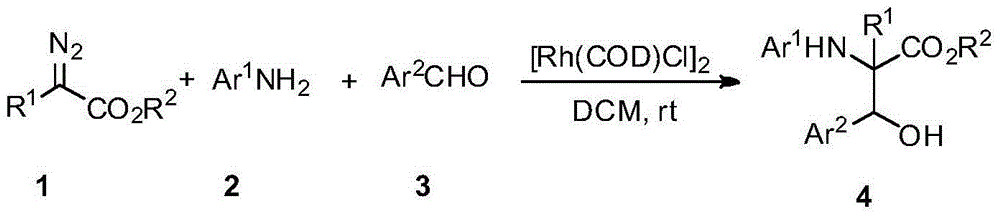
Beta-hydroxy-alpha-amino acid derivative, and synthesis method and application thereof
A technology of derivatives and hydroxy esters, applied in the field of new β-hydroxy-α-amino acid derivatives and their chemical synthesis, can solve the limitation of the scope of reaction substrates, reduce the variety of α-amino-β-hydroxy ester derivatives The problems such as low chemical selectivity and low chemical selectivity can be solved, and the effect of low cost, high diastereoselectivity and simple synthesis route can be achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of compound 4a of the present invention
[0029] First take by weighing p-nitrobenzaldehyde 3a (0.1mmol), o-methoxyaniline 2a (0.12mmol), benzyldiazoethyl ester 1a (0.2mmol), (1,5-cyclooctadiene) rhodium chloride ( I) dimer (5.0 mg, 0.01 mmol), p-nitrobenzaldehyde 3a, (1,5-cyclooctadiene) rhodium(I) chloride dimer and 1 ml dichloromethane were added to a small test tube reactor , let p-nitrobenzaldehyde 3a and (1,5-cyclooctadiene) rhodium chloride (I) dimer dissolve, then, benzyl diazoethyl ester 1a and o-methoxyaniline 2a with 1ml dichloro Methane was dissolved to obtain 1 ml of a mixed solution of benzyldiazoethyl ester 1a and o-methoxyaniline 2a, and then the mixture of benzyldiazoethyl ester 1a and o-methoxyaniline 2a was mixed by a peristaltic pump for 0.5 hours at room temperature The solution was slowly added dropwise to the reaction flask, and after the reaction was completed, the solvent was removed by rotary evaporation at 30°C-40°C to o...
Embodiment 2-13
[0034] Example 2-13 Preparation of Compound β-Hydroxy-α-Amino Acid Derivatives (4b~4m)
[0035]In Examples 2-13, see Table 1 for the changes of substituents, compound numbers, d.r.
[0036] Table 1
[0037]
[0038] The characterization of the product β-hydroxy-α-amino acid derivatives 4b to 4m is as follows:
[0039] Characterization of 4b:
[0040] 1 H NMR (400MHz, CDCl3, 25°C, TMS) δ7.36(d, J=12.0Hz, 2H), 7.14(d, J=12.0Hz, 2H), 7.08(m, 3H), 6.94(m, 2H ), 6.84-6.81(m, 2H), 6.79-6.71(m, 2H), 5.32-5.26(m, 2H), 4.04-3.97(m, 2H), 3.85(d, J=8.0Hz, 1H), 3.72-3.68(m, 4H), 3.53-3.49(d, J=8.0Hz, 1H), 1.05(t, J=8.0Hz, 3H).
[0041] 13 C NMR (400MHz, CDCl3, 25°C, TMS) δ172.54, 147.86, 138.86, 135.66, 134.73, 130.77, 130.19, 128.78, 127.89, 126.68, 121.75, 120.90, 117.75, 113.40, 1140.437, 6 55.78, 37.76.13.75.
[0042] Characterization of 4c:
[0043] 1 H NMR (400MHz, CDCl3, 25°C, TMS) δ7.47-7.40(m, 2H), 7.29(s, 1H), 7.16(d, J=7.4Hz, 4H), 7.00(s, 2H), 6.86 -6.82(m, 2H), 6...
Embodiment 14
[0075] Example 14 Inhibition of protein tyrosine phosphatase activity by β-hydroxy-α-amino acid derivatives 4a, 4b, 4f, 4g, 4k, 4l, 4m of the present invention
[0076] PTP1B is the first identified protein tyrosine phosphatase (protein tyrosine phosphatase). Experiments with PTP1B knockout mice have shown that PTP1B regulates insulin sensitivity and fat metabolism through dephosphorylation of insulin receptors. play a very important role in the process. Therefore, selective and highly active PTP1B inhibitors are of great value in the treatment of diabetes and obesity.
[0077] Screening method:
[0078] Protocol id: 25
[0079] Protocol name: PTP1B activity assay, absorbance
[0080] Instrument: VERSAmax (Molecular Devices, USA).
[0081] Material: PTP1B, our laboratory uses E. coli expression system to obtain GST fusion protein.
[0082] Substrate, pNPP.
[0083] Procedure: Enzyme activity is detected in 96-well or 384-well flat-bottomed clear microplates using light a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com