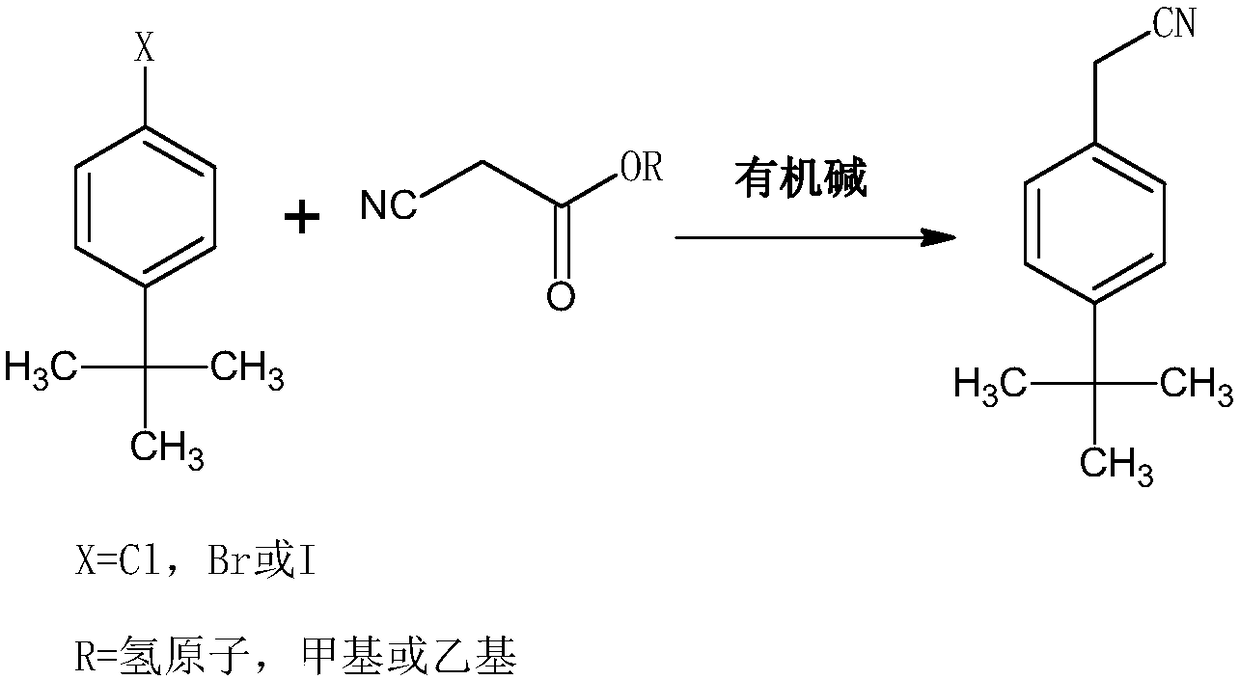
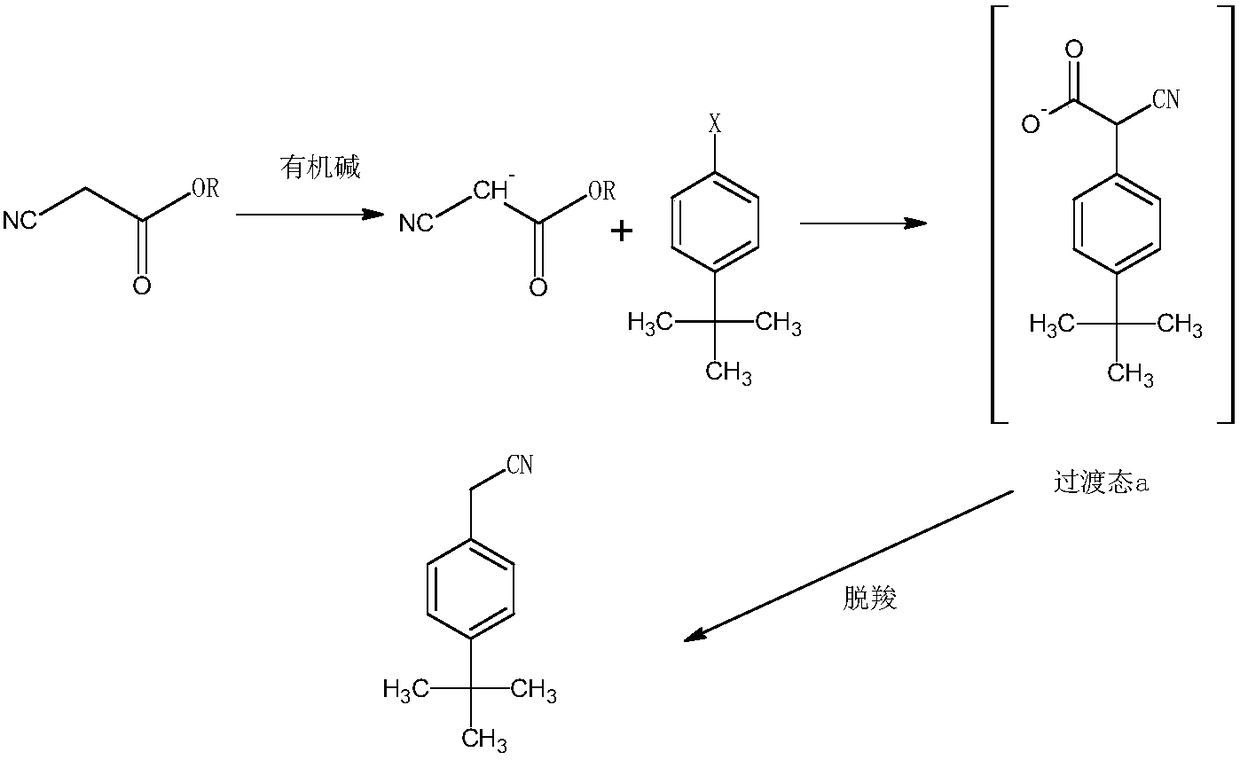
Synthesis method of acaricide cyflumetofen intermediate p-tert-butyl phenylacetonitrile
A technology of tert-butylphenylacetonitrile and cyflufen, which is applied in the field of pesticides and pharmaceuticals, can solve the problems of high and serious distillation residues, environmental pollution, etc., and achieve the effects of high reaction yield, short steps and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic method of tert-butylphenylacetonitrile of acaricide cyflumetate intermediate, comprises the following steps:
[0029] 1) Under the condition of argon, mix ethyl cyanoacetate, potassium tert-butoxide and tert-butanol, raise the temperature to 98°C, control the pressure to 4.5 atmospheres, and mix 1mol of tert-butylchlorobenzene and DMSO within 30min The solution was added in 3 batches, then the reaction temperature was controlled to be 112°C, the reaction pressure was 5 atmospheres, the reaction was completed for 2.5 hours, then deionized water was added, the reaction temperature was controlled to be 142°C, the reaction pressure was 8 atmospheres, and the reaction was continued for 5 hours Finish;
[0030] The molar ratio of 4-tert-butylchlorobenzene to ethyl cyanoacetate is 1:1.35, the molar ratio of ethyl cyanoacetate to potassium tert-butoxide is 1:1.55, and the molar ratio of ethyl cyanoacetate to tert-butanol 1g:10ml, the ratio of 4-tert-butylchlorobe...
Embodiment 2
[0033] The synthetic method of tert-butylphenylacetonitrile of acaricide cyflumetate intermediate, comprises the following steps:
[0034] 1) Under nitrogen, mix methyl cyanoacetate, sodium tert-butoxide, and tetrahydrofuran, heat up to 80°C, control the pressure to 3 atmospheres, and divide 1mol of tert-butylbromobenzene and DMF solution within 20min Add in 3 batches, then control the reaction temperature to 95°C, the reaction pressure to 4 atmospheres, and finish the reaction for 1 hour, then add deionized water, control the reaction temperature to 130°C, and the reaction pressure to 6 atmospheres, and continue the reaction for 4 hours to end;
[0035] The molar ratio of 4-tert-butylbromobenzene to methyl cyanoacetate is 1:1.2, the molar ratio of methyl cyanoacetate to sodium tert-butoxide is 1:1.4, and the molar ratio of methyl cyanoacetate to tetrahydrofuran is 1g : 8ml, the consumption ratio of 4-tert-butylbromobenzene and DMF is 1g: 4ml, and the consumption ratio of sodi...
Embodiment 3
[0038] The synthetic method of tert-butylphenylacetonitrile of acaricide cyflumetate intermediate, comprises the following steps:
[0039] 1) Under argon atmosphere, mix isopropyl cyanoacetate, potassium tert-butoxide and tert-butanol, heat up to 105°C, control the pressure to 5 atmospheres, and mix 1mol of tert-butyl iodobenzene and Add the DMSO solution in 3 batches, then control the reaction temperature to 120°C, the reaction pressure to 7 atmospheres, and the reaction is over for 3 hours, then add deionized water, control the reaction temperature to 150°C, and the reaction pressure to 9 atmospheres, and continue the reaction for 6 hours Finish;
[0040] The consumption ratio of 4-tert-butyl iodobenzene and isopropyl cyanoacetate is a molar ratio of 1:1.45, the consumption of isopropyl cyanoacetate and organic base is a molar ratio of 1:1.62, and the consumption ratio of isopropyl cyanoacetate and DMSO is 1g:12ml, the ratio of 4-tert-butyl iodobenzene to DMSO is 1g:6ml, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com