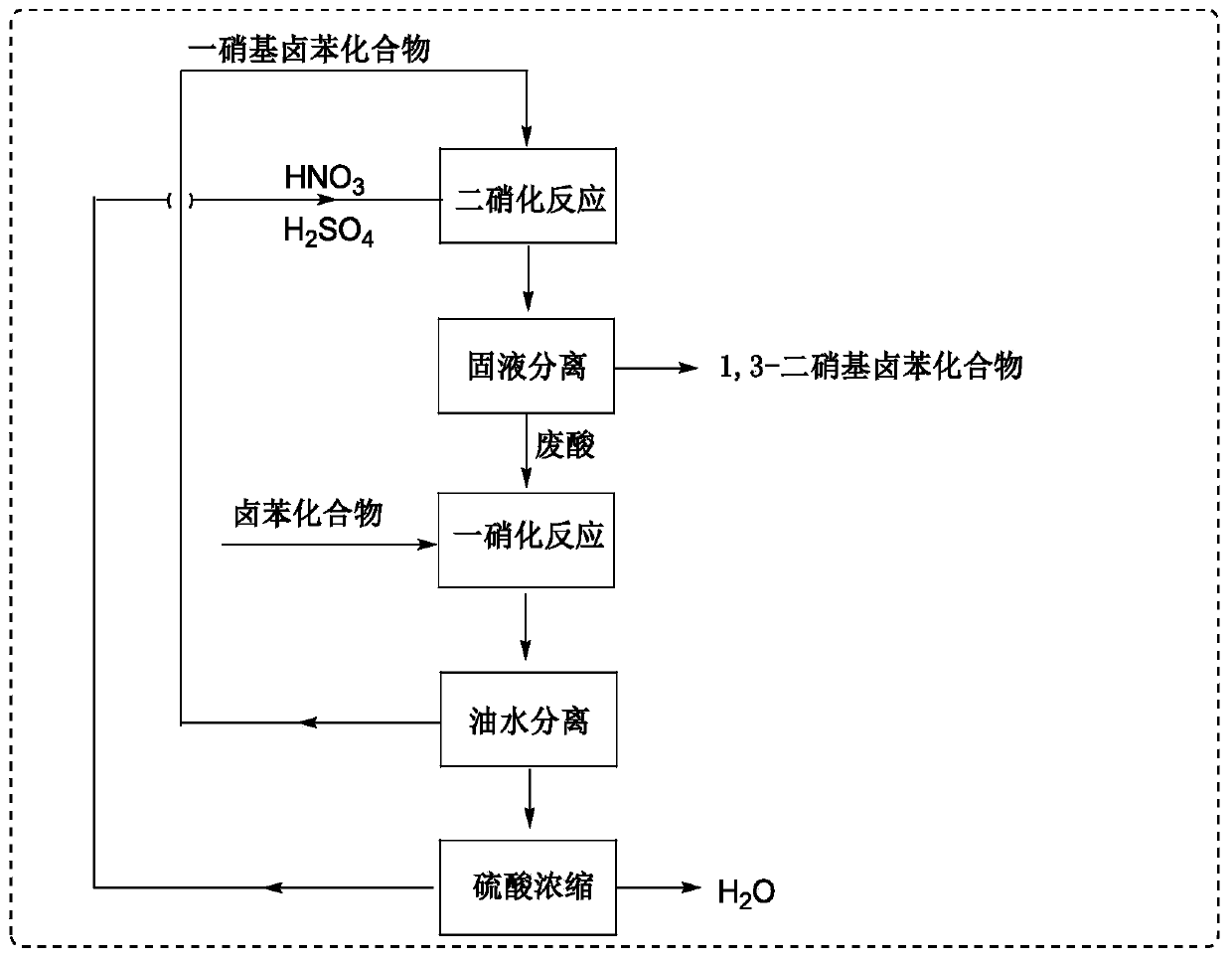
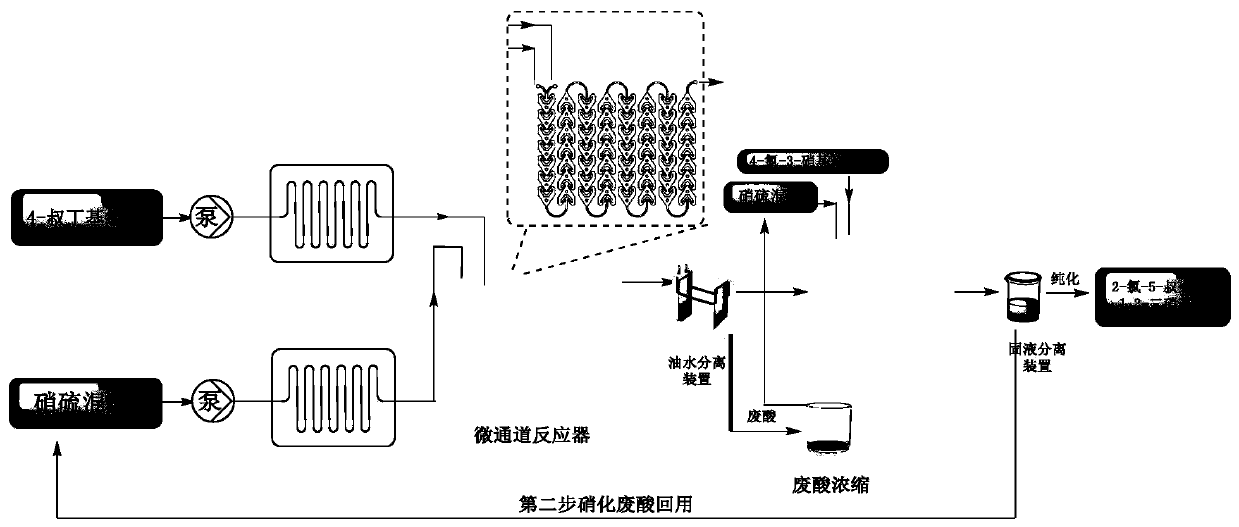
Method for synthesizing 1,3-dinitrohalobenzene compound
A synthesis method and compound technology, applied in the preparation of nitro compounds, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of severe exothermic heat of nitration reaction, large content of nitric acid, high cost of waste sulfuric acid treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] 1000 g (5.93 mol) of 4-tert-butylchlorobenzene was dissolved in 250 g of 1,2-dichloroethane to prepare a 4-tert-butylchlorobenzene solution. 2000 grams (20 moles) of 95%-98% sulfuric acid are added dropwise to 643 grams (10 moles) of 95%-98% nitric acid dissolved in a 2 liter three-neck flask kept warm in an ice-water bath to prepare the mixed acid of nitric acid sulfuric acid .
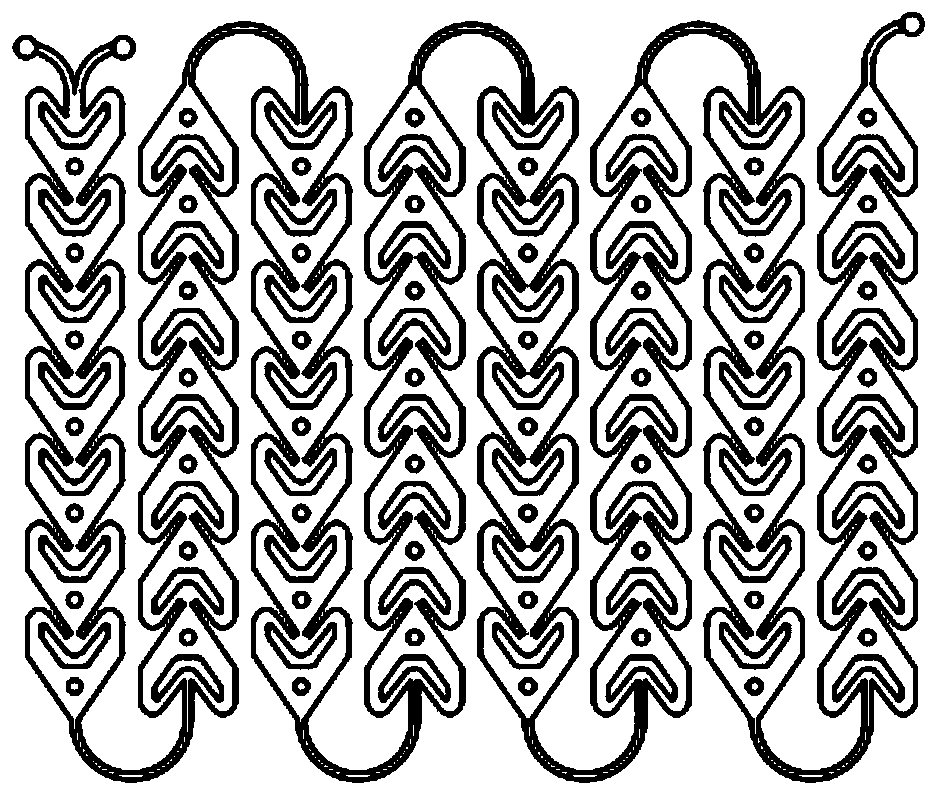
[0098] The first nitration reaction: according to the molar ratio of 4-tert-butyl chlorobenzene and nitric acid 1:1.2, the flow rate of 4-tert-butyl chlorobenzene is 2.7ml / min, the flow rate of mixed acid is 2.8ml / min, and the residence time is preferred For 152s, the reaction temperature is 60-80°C, part of the 4-tert-butylchlorobenzene solution and the mixed acid of nitric acid and sulfuric acid are pumped through the continuous flow microreactor, which includes separate fluid modules connected in sequence, specifically the inlet mixed reaction module , 8 resident modules and one export mod...
Embodiment 2
[0113] 1000 g (5.93 mol) of 4-tert-butylchlorobenzene was dissolved in 250 g of 1,2-dichloroethane to prepare a 4-tert-butylchlorobenzene solution. 2000 grams (20 moles) of 95%-98% sulfuric acid are added dropwise to 643 grams (10 moles) of 95%-98% nitric acid dissolved in a 2 liter three-neck flask kept warm in an ice-water bath to prepare the mixed acid of nitric acid sulfuric acid .
[0114] The first nitration reaction: according to the molar ratio of 4-tert-butylchlorobenzene and nitric acid 1:1.2, the reaction temperature is 60-70°C, the flow rate of 4-tert-butylchlorobenzene is 2.6mL / min, and the flow rate of mixed acid is 2.7mL / min , part of the mixed acid of 4-tert-butylchlorobenzene solution and nitric acid sulfuric acid is pumped through a continuous flow microreactor, which includes separate fluid modules connected in sequence, specifically an inlet mixed reaction module, 8 resident modules and an outlet module , as above. Each individual fluidic module was maint...
Embodiment 3
[0122] 1000 g (5.93 mol) of 4-tert-butylchlorobenzene was dissolved in 250 g of 1,2-dichloroethane to prepare a 4-tert-butylchlorobenzene solution. 2000 grams (20 moles) of 95%-98% sulfuric acid are added dropwise to 643 grams (10 moles) of 95%-98% nitric acid dissolved in a 2 liter three-neck flask kept warm in an ice-water bath to prepare the mixed acid of nitric acid sulfuric acid .
[0123] The first nitration reaction: according to the molar ratio of 4-tert-butylchlorobenzene and nitric acid 1:1.2, the reaction temperature is 60-80°C, the flow rate of 4-tert-butylchlorobenzene is 2.5mL / min, and the flow rate of mixed acid is 2.6mL / min , part of the mixed acid of 4-tert-butylchlorobenzene solution and nitric acid sulfuric acid is pumped through a continuous flow microreactor, which includes separate fluid modules connected in sequence, specifically an inlet mixed reaction module, 8 resident modules and an outlet module , as above. Each individual fluidic module was maint...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com